It is possible to write simple equations to relate pH, pK, and concentrations (c) of various solutions.
Question:
It is possible to write simple equations to relate pH, pK, and concentrations (c) of various solutions. Three such equations are shown here.
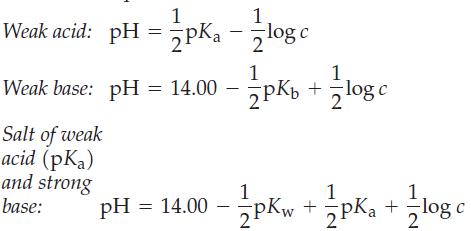
(a) Derive these three equations, and point out the assumptions involved in the derivations.
(b) Use these equations to determine the pH of 0.10 M CH3COOH(aq), 0.10 M NH3(aq), and 0.10 M NaCH3COO(aq).
Verify that the equations give correct results by determining these pH values in the usual way.
Transcribed Image Text:
1 Weak acid: pH = pK₁ - 1log c 1 Weak base: pH = 14.00 --- pKb 2PK₂ Salt of weak acid (pka) and strong base: +=log c 1 1 pH = 14.00 - 2pKw+ Pka + log c
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 0% (1 review)
Lets derive and discuss the three given equations for weak acids weak bases and salts of weak acids and strong bases After that well use these equatio...View the full answer

Answered By

Asim farooq
I have done MS finance and expertise in the field of Accounting, finance, cost accounting, security analysis and portfolio management and management, MS office is at my fingertips, I want my client to take advantage of my practical knowledge. I have been mentoring my client on a freelancer website from last two years, Currently I am working in Telecom company as a financial analyst and before that working as an accountant with Pepsi for one year. I also join a nonprofit organization as a finance assistant to my job duties are making payment to client after tax calculation, I have started my professional career from teaching I was teaching to a master's level student for two years in the evening.
My Expert Service
Financial accounting, Financial management, Cost accounting, Human resource management, Business communication and report writing. Financial accounting : • Journal entries • Financial statements including balance sheet, Profit & Loss account, Cash flow statement • Adjustment entries • Ratio analysis • Accounting concepts • Single entry accounting • Double entry accounting • Bills of exchange • Bank reconciliation statements Cost accounting : • Budgeting • Job order costing • Process costing • Cost of goods sold Financial management : • Capital budgeting • Net Present Value (NPV) • Internal Rate of Return (IRR) • Payback period • Discounted cash flows • Financial analysis • Capital assets pricing model • Simple interest, Compound interest & annuities
4.40+
65+ Reviews
86+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
Do some amendment and enhance the given research paper: Table of Content Abstract..3 Action Research.4 Research Methodology and Design...5 Literature Review: NoSQL Database7 Proposal.7 Iteration 1..8...
-
A sample containing an alkali sulfate is dried, weighed and dissolved in dilute HCl. Barium chloride solution is added in excess to precipitate barium sulfate, and the precipitate is digested in the...
-
In February 19_8, Randy White, president of Arriscraft Corporation (Arriscraft), had just received two requests for a price on two of their marble products. The first request was from a nearby city...
-
Alpha Appliance Service had net income for the year of $ 35,000. In addition, the balance sheet reports the following balances: Calculate the return on assets (ROA) for Alpha Appliance Service for...
-
Using the information given in Exercise 1626 and assuming pretax financial income of $3,100,000, calculate taxable income.
-
On August 1 of the current year, a business paid the August rent on the building that it occupies. (a) Do the rights acquired at August 1 represent an asset or an expense? (b) What is the...
-
Plaintiffs purchased stock warrants (rights to purchase) for blocks of Osborne Computer Corp., the manufacturer of the first mass-market portable personal computer. Because of inability to produce a...
-
As loan analyst for Utrillo Bank, you have been presented the following information. Each of these companies has requested a loan of $50,000 for 6 months with no collateral offered. Because your bank...
-
The Oracle Database Upgrade Assistant (DBUA) is a GUI tool but can also run in silent command line mode. As a DBA, in what circumstances would you recommend using silent mode?
-
For the ionization of phenylacetic acid, CHCHCOH + HO H3O+ + CH5CHCO Ka 4.9 x 10-5 = (a) What is [C6H5CHCO2] in 0.186 M C6H5CHCOH? (b) What is the pH of 0.121 M C6H5CHCOH?
-
For H 2 SO 3 (aq), K a1 = 1.3 x 10 -2 and K a2 = 6.3 x 10 -8 . In 0.10 M H 2 SO 3 (aq), (a) [HSO3] = 0.013 M; (b) [SO3] = 6.3 108 M; (c) [H3O] = 0.10 M; (d) [H3O] = 0.013 M; (e) [SO3-] = 0.036 M.
-
An investment costing $90,000 is being contemplated by Mergenthaler Inc. The investment will have a life of 8 years with no salvage value and will produce annual cash flows of $16,870. Instructions...
-
Short selling works by... Manipulating interest rates Using foreign stock markets to diversify Betting against the housing market Borrowing and selling shares, then buying back later
-
How would you invest in a SPAC? As an individual investor. how would you perform fundamental analysis prior to your investment in a SPAC? How would you invest in a Direct Listing? As an individual...
-
Lab 11: Classes and methods 3 Introduction In this lab, we continue working on classes and methods. The work is based on the previous lab, the Stat class. You will use existing code that you should...
-
Briefly describe ASCII and Unicode and draw attention to any relationship between them. [3 marks] (b) Briefly explain what a Reader is in the context of reading characters from data. [3 marks] A...
-
All of the following statements concerning the PEG (Price-Earnings-Growth) ratio are correct, EXCEPT: a. PEG equals 1 indicates a fairly priced stock b. PEG over 1 indicates an underpriced stock ...
-
Refer to the situation described in BE 10-14. Assuming the company uses the weighted-average method, calculate the amount of interest capitalized for the year.
-
Difference between truncate & delete
-
J. Lynn, M. Oller, and F. Tate share income on a 5 : 3 : 2 basis. They have capital balances of $30,000, $26,000, and $18,000, respectively, when Doc Duran is admitted to the partnership....
-
G. Olde and R. Young share income on a 6 : 4 basis. They have capital balances of $100,000 and $70,000, respectively, when K.Twener is admitted to the partnership. Instructions Prepare the journal...
-
B. Cates, V. Elder, and S. Nguyen have capital balances of $50,000, $40,000, and $32,000, respectively. Their income ratios are 5 : 3 : 2. Nguyen withdraws from the partnership under each of the...
-
Find the critical values x + -a/2 and x a/2 for a 98% confidence level and a sample size of n = 30. x-a/2 = (Round to three decimal places as needed.) X2 = (Round to three decimal places as needed.)
-
On the Navajo Reservation, a random sample of 214 permanent dwellings in the Fort Defiance region showed that 52 were traditional Navajo hogans. In the Indian Wells region, a random sample of 139...
-
It is reported that female students spend more than 30 minutes daily doing make-up. A researcher believes the average time spend on make-up is lower. i) Hypothesis (H0 and H1) ii) One-tailed (upper...

Study smarter with the SolutionInn App


