Lead metal is added to 0.100 M Cr 3+ (aq). What are [Pb 2+ ], [Cr 2+
Question:
Lead metal is added to 0.100 M Cr3+(aq). What are [Pb2+], [Cr2+], and [Cr3+] when equilibrium is established in the reaction?
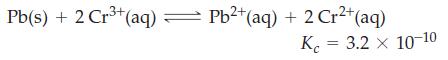
Transcribed Image Text:
3+ Pb(s) + 2 Cr³+ (aq) = Pb²+ (aq) + 2 Cr²+ (aq) K 3.2 x 10-10 =
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (2 reviews)
The reaction is as follows Pbs 2 Cr3aq Pb2aq 2 Cr2aq The equilibrium constant is Kc 32 ...View the full answer

Answered By

Joseph Mwaura
I have been teaching college students in various subjects for 9 years now. Besides, I have been tutoring online with several tutoring companies from 2010 to date. The 9 years of experience as a tutor has enabled me to develop multiple tutoring skills and see thousands of students excel in their education and in life after school which gives me much pleasure. I have assisted students in essay writing and in doing academic research and this has helped me be well versed with the various writing styles such as APA, MLA, Chicago/ Turabian, Harvard. I am always ready to handle work at any hour and in any way as students specify. In my tutoring journey, excellence has always been my guiding standard.
4.00+
1+ Reviews
10+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
When magnesium metal is added to a beaker of HCl(aq), a gas is produced. Knowing that magnesium is oxidized and that hydrogen is reduced, write the balanced equation for the reaction. How many...
-
An excess of zinc metal is added to 50.0 mL of a 0.100 M AgNO3 solution in a constant-pressure calorimeter like the one pictured in Figure 6.9. As a result of the reaction The temperature rises from...
-
Let us explore a reaction with a limiting reactant. Here, zinc metal is added to a flask containing aqueous HCl, and H 2 gas is a product. The three flasks each contain 0.100 mol of HCl. Zinc is...
-
Herbs Pty Ltd is considering investing in a new herb packaging machine. The machine is estimated to cost $80,000 which can last for 7 years before it becomes too costly to maintain and can be sold...
-
Wells and Associates has EBIT of $67,500. Interest costs are $22,500, and the firm has 15,000 shares of common stock outstanding. Assume a 40% tax rate. a. Use the degree of financial leverage (DFL)...
-
Financial statement information about four different companies is shown below. Instructions (a) Determine the missing amounts. (Hint: For example, to solve for (a), Assets Liabilities =...
-
Mikes Powersports uses the LIFO inventory method. Mikes Powersports started August with 10 helmets that cost \($54\) each. On August 19, Mikes Powersports bought 15 helmets at \($56\) each. On August...
-
A municipality expects to use a landfill evenly throughout the 25 years from January 1, 2017, to December 31, 2041. Upon closing the landfill it estimates that it will incur closing costs of...
-
The S&L Corporation is producing two types of candy bars: Mint and Orange. Relevant data on the product demand and bottleneck operation for the upcoming fiscal year are given in the following table:...
-
One sketch below represents an initial nonequilibrium mixture in the reversible reaction Which of the other three sketches best represents an equilibrium mixture? Explain. Kc = 4.0 (8) (8) + (8)
-
One sketch below represents an initial nonequilibrium mixture in the reversible reaction Which of the other three sketches best represents an equilibrium mixture? Explain. 2 NO(g) + Br(g) = 2 NOBr(g)...
-
Review current money market rates.
-
Please solve a) to i) according to the graph given using Microsoft Excel and provide Excel screenshot You have been asked to analyze two mutually exclusive projects. Expected Cash Flows Project A a)...
-
4 Part A A21-g rifle bullet traveling 210 m/s buries itself in a 3.2-kg pendulum hanging on a 22-m-long string, which makes the pendulum swing upward in an arc Determine the vertical and horizontal...
-
Miller Company's contribution format income statement for the most recent month is shown below: Total Sales (25,200 units) Variable expenses $ 226,800 136,080 Contribution margin 90,720 Per Unit $...
-
Head-First Company plans to sell 5,120 bicycle helmets at $74 each in the coming year. Unit variable cost is $45 (includes direct materials, direct labor, variable factory overhead, and variable...
-
What trends are important in the health industry? What trends are important in the hygiene industry?
-
Robin Thomas, the owner of Thomas Custom Cabinets, is preparing a bid on a kitchen remodeling job for Matt and Heather. Robin expects that the job will require $1,700 of direct materials, $2,800 of...
-
Is the modified 5-question approach to ethical decision making superior to the modified moral standards or modified Past in approach?
-
McLean Company produces a product that requires three standard gallons per unit. The standard price is $18.50 per gallon. If 2,500 units required 8,000 gallons, which were purchased at $18.00 per...
-
Norris Company produces a product that requires 3.5 standard hours per unit at a standard hourly rate of $12 per hour. If 500 units required 1,500 hours at an hourly rate of $11.50 per hour, what is...
-
McLean Company produces a product that requires two standard hours per unit at a standard hourly rate of $18 per hour. If 2,500 units required 5,500 hours at an hourly rate of $19 per hour, what is...
-
In Anglo-Saxon countries, such as the United States and England, the mechanisms that are established to regulate the activity of auditors within the framework of professional self-regulation, while...
-
Crane Company is considering a capital investment of $369,600 in additional productive facilities. The new machinery is expected to have useful life of 6 years with no salvage value. Depreciation is...
-
During Heaton Company's first two years of operations, it reported absorption costing net operating income as follows: Year 1 Sales (@$64 per unit) Cost of goods sold (@ $40 per unit) Gross margin $...

Study smarter with the SolutionInn App


