Lead(IV) oxide, PbO 2 , is a good oxidizing agent. Use appropriate data from Appendix D to
Question:
Lead(IV) oxide, PbO2, is a good oxidizing agent. Use appropriate data from Appendix D to determine whether PbO2(s) in a solution with [H3O+] = 1 M is a sufficiently good oxidizing agent to carry the following oxidations to the point at which the concentration of the species being oxidized decreases to one-thousandth of its initial value.
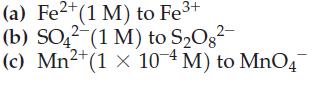
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:





