Ozone is a powerful oxidizing agent. Using ozone as the oxidant, write equations to represent oxidation of
Question:
Ozone is a powerful oxidizing agent. Using ozone as the oxidant, write equations to represent oxidation of
(a) Br–(aq) to BrO–(aq) (hypobromite);
(b) NO(g) to NO2(g);
(c) Fe2+(aq) to Fe3+(aq) in acidic solution;
(d) Ag(s) to AgO(s);
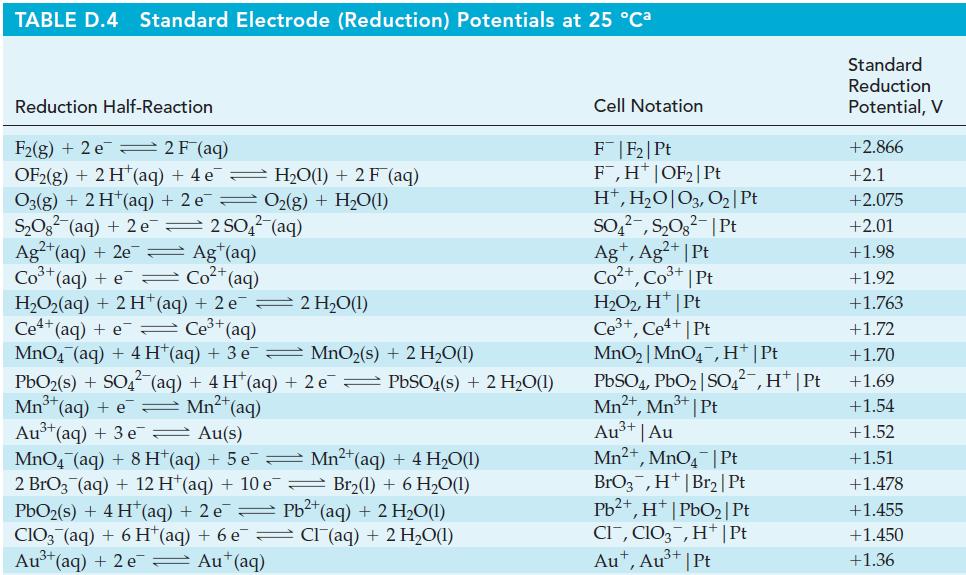
(e) [Fe(CN)6]4– to [Fe(CN)6]3– in basic solution. The half-equations for the reduction of O3 in acidic and basic solutions are given in Table D.4 of Appendix D.
Table D.4
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:





