Reaction (20.16) is started with pure DTBP at 147 C and 800.0 mmHg pressure in a flask
Question:
Reaction (20.16) is started with pure DTBP at 147 °C and 800.0 mmHg pressure in a flask of constant volume.
(a) What is the value of the rate constant k?
(b) At what time will the partial pressure of DTBP be 50.0 mmHg?
Reaction (20.16)
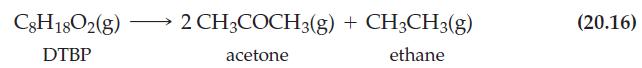
Transcribed Image Text:
C8H1802(g) DTBP 2 CH3COCH3(g) + CH3CH3(g) acetone ethane (20.16)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (3 reviews)
Analyze In part a we observe from Figure 205 that t 12 80 x 1...View the full answer

Answered By

Utsab mitra
I have the expertise to deliver these subjects to college and higher-level students. The services would involve only solving assignments, homework help, and others.
I have experience in delivering these subjects for the last 6 years on a freelancing basis in different companies around the globe. I am CMA certified and CGMA UK. I have professional experience of 18 years in the industry involved in the manufacturing company and IT implementation experience of over 12 years.
I have delivered this help to students effortlessly, which is essential to give the students a good grade in their studies.
3.50+
2+ Reviews
10+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
Refer to Example 20-7. For the decomposition of di-tbutyl peroxide (DTBP), determine the time at which the total gas pressure is 2100 mmHg. Example 20-7 Reaction (20.16) is started with pure DTBP at...
-
A saturated solution of sucrose (C12H22O11) is made by dissolving excess table sugar in a flask of water. There are 50 g of un-dissolved sucrose crystals at the bottom of the flask in contact with...
-
The equilibrium reaction N 2 O 4 (g) 2 NO 2 (g) has been thoroughly studied (Figure 15.7). (a) If the total pressure in a flask containing NO 2 and N 2 O 4 gas at 25C is 1.50 atm and the value of K...
-
Data Set 14 in Appendix B lists highway fuel consumption amounts (mi/gal) for cars categorized by size (small, midsize, large). If we use those highway fuel consumption amounts arranged into the...
-
Franklin Company purchased a machine on January 1, 2008, paying $150,000. The machine was estimated to have a useful life of eight years and an estimated salvage value of $30,000. In early 2010, the...
-
Consider a bond that promises the following cash flows. The yield to maturity is 12%. You plan to buy this bond, hold it for 2.5 years, and then sell the bond. a. What total cash will you receive...
-
If your instructor assigns a marketing plan for your class, we hope you will be excitedfor two reasons. First, you will get insights into trying to actually do marketing that often go beyond what you...
-
The partners in New Yorker Company decide to liquidate the firm when the balance sheet shows the following The partners share income and loss 5 : 3 : 2. During the process of liquidation, the...
-
What other options exist if you do not want to use the relational model? Researchon the Internet and find one other type of database model, describe that model, and post the link to the source that...
-
If the rate of reaction (20.3) is 5.7 x 10 4 M s 1 , what is the rate of production of O 2 (g) from 1.00 L of the H 2 O 2 (aq), expressed as (a) mol O2 s 1 ; (b) mol O 2 min 1 ; (c) mL O 2 (STP) min...
-
Use a value of k = 7.30 x 10 -4 s -1 for the first-order decomposition of H 2 O 2 (aq) to determine the percent H 2 O 2 that has decomposed in the first 500.0 s after the reaction begins.
-
The acceleration of a particle is directly proportional to the square of the time t. When t = 0, the particle is at x = 36 ft. Knowing that at t = 9 s, x = 144 ft and v = 27 ft/s, express x and v in...
-
Write a memo to your CFO. Include discussion of each of these points: How the sale portion of the sale-leaseback transaction should be accounted for at the lease's inception. How the gain on the sale...
-
Durham, Inc. issued $500,000 of its 10-year zero-coupon bonds on January 1, Year 6, to yield 9%. The effective interest method is used. PV of $1 (9%) PV of an Annuity of $1 (9%) FV of $1 (9%) FV of...
-
Explain the below general Information Retrieval (IR) system architecture - user feedback Ranked documents The user User query Query operations Executable query Retrieval system Document collection...
-
A right-hand circularly polarized, uniform plane wave E,His obliquely incident on the planar interface between two non-magnetic dielectric media as shown in the above figure. It is observed that the...
-
Carole Lemieux works for The Wellness Centre in Ajax, Ontario. Carole is paid on a semi-monthly basis. Her gross pensionable/taxable income per pay is $1,575.00. Please calculate CPP.
-
At the beginning of 2011, Quentin and Kopps (Q&K) adopted the dollar-value LIFO (DVL) inventory method. On that date the value of its one inventory pool was $84,000. The company uses an internally...
-
The following cost information was provided to you for analysis: September 12,000 Units Produced Costs: TIC TAC TOE TING August 10,000 P80,000 70.000 60.000 50,000 How much is the fixed cost per...
-
The static manufacturing overhead budget based on 40,000 direct labor hours shows budgeted indirect labor costs of $54,000. During March, the department incurs $65,000 of indirect labor while working...
-
A static overhead budget based on 40,000 direct labor hours shows Factory Insurance $6,500 as a fixed cost. At the 50,000 direct labor hours worked in March, factory insurance costs were $6,200. Is...
-
Kate Coulter is confused about how a flexible budget is prepared. Identify the steps for Kate.
-
Discuss four broad activities that erode the value of the kenyan currency. State key financial disciples that would reduce the pressure for foreign currency
-
, we add a strong acid, HCl or H2SO4, to a strong base, NaOH, in the calorimeter. Both the acid and base are diluted in water. Once the acid and base are mixed together, the acid and base completely...
-
Answer question #2. Thanks 1. (See BKM Chapter 7.2, Equations 7.2 and 7.7 for this question). Stock A's expected return and standard deviation are E[RA] = 8% and A= 15%, while stock B's expected...

Study smarter with the SolutionInn App


