Refer to Example 23-2. Select a reducing agent (from Table 23.1 or Appendix D) that will reduce
Question:
Refer to Example 23-2. Select a reducing agent (from Table 23.1 or Appendix D) that will reduce VO2+ to V3+ and no further in acidic solution.
Example 23-2
Can MnO4–(aq) be used to oxidize VO2+(aq) to VO2+(aq) for standard-state conditions in an acidic solution? If so, write a balanced equation for the redox reaction.
Table 23.1
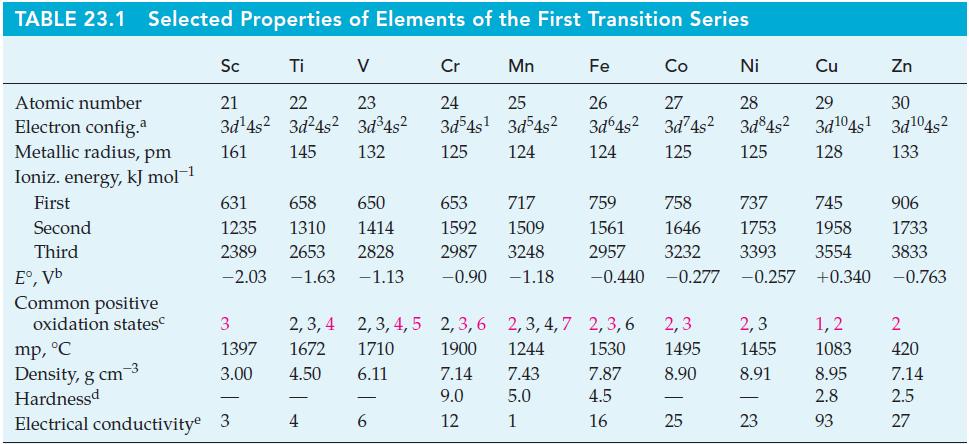
Transcribed Image Text:
TABLE 23.1 Selected Properties of Elements of the First Transition Series Atomic number Electron config.a Metallic radius, pm Ioniz. energy, kJ mol-1 First Second Third E°,Vb Common positive oxidation states Sc Ti V 21 22 3d¹4s² 3d²4s² 161 145 3 1397 3.00 mp, °C Density, g cm-3 Hardnessd Electrical conductivity 3 23 3d³4s² 132 631 658 650 653 717 1235 1414 1592 1509 1310 2389 2653 2828 2987 3248 -2.03 -1.63 -1.13 -0.90 -1.18 2,3,4 2,3,4,5 1672 1710 4.50 6.11 4 Cr 24 3d³4s¹ 125 6 Mn 25 3d54s² 124 Fe Co 26 27 3d64s² 3d74s² 124 125 Ni 28 3d84s² 125 759 758 737 745 906 1646 1753 1958 1733 1561 2957 3232 3393 3554 3833 -0.440 -0.277 -0.257 +0.340 -0.763 2,3,6 2,3,4,7 2,3,6 2,3 2,3 1900 1244 1530 1495 1455 7.14 7.43 7.87 8.90 8.91 9.0 5.0 4.5 12 1 16 25 - Cu Zn 29 30 3d¹04s¹ 3d¹04s² 128 133 23 1,2 1083 8.95 2.8 93 2 420 7.14 2.5 27
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (6 reviews)
Reducing agent Zn Balanced equation VO2aq Zns 2 Haq V3aq Zn2aq H2Ol Zn is a go...View the full answer

Answered By

Shubhradeep Maity
I am an experienced and talented freelance writer passionate about creating high-quality content. I have over five years of experience working in the field and have collaborated with several renowned companies and clients in the SaaS industry.
At Herman LLC, an online collective of writers, I generated 1,000+ views on my content and created journal content for 100+ clients on finance topics. My efforts led to a 60% increase in customer engagement for finance clients through revamping website pages and email interaction.
Previously, at Gerhold, a data management platform using blockchain, I wrote and published over 50 articles on topics such as Business Finance, Scalability, and Financial Security. I managed four writing projects concurrently and increased the average salary per page from $4 to $7 in three months.
In my previous role at Bernier, I created content for 40+ clients within the finance industry, increasing sales by up to 40%.
I am an accomplished writer with a track record of delivering high-quality content on time and within budget. I am dedicated to helping my clients achieve their goals and providing exceptional results.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
(A) Use data from Tables 19.1 and 23.4 to determine whether nitric acid can be used to oxidize V 3+ (aq) to VO 2+ (aq) for standard-state conditions. If so, write a balanced equation for the...
-
Managing Scope Changes Case Study Scope changes on a project can occur regardless of how well the project is planned or executed. Scope changes can be the result of something that was omitted during...
-
Case Study: Quick Fix Dental Practice Technology requirements Application must be built using Visual Studio 2019 or Visual Studio 2017, professional or enterprise. The community edition is not...
-
You are in a supermarket, shopping for orange juice. There are several brands of orange juice on the shelf. Provide one example of how you may use the availability heuristic to decide which brand of...
-
Income Statement Calculations Required: Complete the income statement calculations by filling in all missingnumbers. Company A Company B Company C Company C $1,310 600 249 Sales revenue $2,000 (4)...
-
Transactions follow for Cassio Limited: March 10 Purchased goods billed at $40,000, terms 3/10, n/60. 11 Purchased goods billed at $25,000, terms 1/15, n/30. 19 Paid invoice of March 10. 24 Purchased...
-
5. Why do fast-growing companies typically fail to sustain their high growth rates?
-
Speedy Spuds is a fast-food restaurant offering all kinds of potatoes. The manager has a 30-second rule for serving customers. Servers at the counter say they could achieve that rule if the form they...
-
Question no. 3 Mr. Rakib is a licensed CPA. During the first month of operations of his business, the following events and transactions occurred during the period. June 1 Shakib invested Tk. 75,000...
-
Based on the description of the nickelcadmium cell, and with appropriate data from Appendix D, estimate E for the reduction of NiO(OH) to Ni(OH) 2 . TABLE D.1 Ground-State Electron Configurations...
-
Write plausible half-equations to represent each of the following in basic solution. (a) Oxidation of Fe(OH) 3 (s) to FeO 4 2 ; (b) Reduction of [Ag(CN) 2 ] to silver metal.
-
The balance in the unearned fees account, before adjustment at the end of the year, is $23,676. Journalize the adjusting entry required assuming the amount of unearned fees at the end of the year is...
-
The adjusted trial balance section of Menlo Company's worksheet shows a \(\$ 1,500\) debit balance in utility expense. At the end of the accounting period the accounting manager accrues an additional...
-
Identify each of the 10 amount columns of the worksheet and indicate to which column the adjusted balance of the following accounts would be extended: a. Accounts Receivable b. Accumulated...
-
Using the data from Table 3.3, show the effect on world output if each country moved toward specialization in the production of its comparative-disadvantage good. TABLE 3.3 Comparative Advantage as a...
-
The Professional Winner was RJ Andrews from Info We Trust, for the video Are Gazelles Endangered? (a) Watch this video. What data are this video conveying? (b) You can interact with the data and...
-
(a) Draw a simplified ray diagram showing the three principal rays for an object located outside the focal length of a converging lens. (b) Is the image real or virtual? (c) Is it upright or...
-
Let H and K be subgroups of a group G, where e is the identity of G. Prove that if |H| = 10 and |K| = 21, then H K = {e}.
-
Draw a Feynman diagram for the reaction n + v p + .
-
Reiden Inc. issues $4 million, 5-year, 8% bonds at 102, with interest payable on January 1. The straight-line method is used to amortize bond premium. (a) Prepare the journal entry to record the sale...
-
Presented below is the partial bond discount amortization schedule for Syam Corp., which uses the effective-interest method of amortization. Instructions(a) Prepare the journal entry to record the...
-
Pickeril Inc. issues a $600,000, 10%, 10-year mortgage note on December 31, 2011, to obtain financing for a new building. The terms provide for semiannual installment payments of $48,145. Prepare the...
-
Your company produces a health magazine. Its sales data for 1 - year subscriptions are as follows: Year of Operation Subscriptions Sold % Expired at Year End 2 0 2 0 $ 3 0 0 , 0 0 0 5 2 0 2 1 $ 6 4 7...
-
Problem 3 - 2 0 ( Static ) Calculate profitability and liquidity measures LO 3 - 3 , 3 - 4 , 3 - 6 Presented here are the comparative balance sheets of Hames Incorporated at December 3 1 , 2 0 2 3...
-
3 Required information [The following information applies to the questions displayed below) John and Sandy Ferguson got married eight years ago and have a seven-year-old daughter. Samantha. In 2020,...

Study smarter with the SolutionInn App


