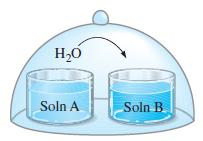
Refer to Figure 14-20(a). Initially, solution A contains 0.515 g urea, CO(NH 2 ) 2 , dissolved
Question:
Refer to Figure 14-20(a). Initially, solution A contains 0.515 g urea, CO(NH2)2, dissolved in 92.5 g H2O; solution B contains 2.50 g sucrose, C12H22O11, dissolved in 85.0 g H2O. What are the compositions of the two solutions when equilibrium is reached, that is, when the two have the same vapor pressure?
Figure 14-20(a)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:





