Starting with SO 3 (g) at 1.00 atm, what will be the total pressure when equilibrium is
Question:
Starting with SO3(g) at 1.00 atm, what will be the total pressure when equilibrium is reached in the following reaction at 700 K?
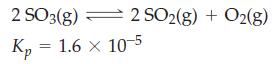
(for pressures in atmospheres).
Transcribed Image Text:
2SO3(g) Кр - 1.6 × 10-5 = 2 SO2(g) + O2(g)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (3 reviews)
The given reaction is 2SO3g2SO2g O2g with Kp 16 105 At equilibrium according to the stoichiometry of ...View the full answer

Answered By

Amit Choudhary
I'm new in this profession regarding online teaching but previously i used to teach students near my college. I am teaching on online platform since last year and got good support from the students. I'm teaching on platforms like chegg and vedantu and also at my home in free time.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
Newsman Co. made the following errors in counting its year-end physical inventories: 2012 ............................................................. $ 60,000 overstatement 2013...
-
Simple Metal Works, Inc. will manufacture and sell 300,000 units next year. Fixed costs will total $350,000, and variable costs will be 65 percent of sales. a. The firm wants to achieve a level of...
-
Refer to Example 15-2. H 2 S(g) at 747.6 mmHg pressure and a 1.85 g sample of I 2 (s) are introduced into a 725 mL flask at 60 C. What will be the total pressure in the flask at equilibrium? Example...
-
The McGraw Company is accumulating data to be used in preparing its annual profit plan for the coming year. The cost behavior pattern of the maintenance costs must be determined. The accounting staff...
-
The current stockholders equity account for Hilo Farms is as follows: Common stock (50,000 shares at $3 par) ... $150,000 Paid-in capital in excess of par .................... 250,000 Retained...
-
Blueline Tours, Inc., operates tours throughout the United States. A study has indicated that some of the tours are not profitable, and consideration is being given to dropping these tours to improve...
-
Plaintiff visited South Chicago on January 10, 2008, seeking a new 2008 Nissan Versa (Versa) with manual transmission, anti-lock brakes, and other features. He was told by the employees of South...
-
Accountants for Carlson, Inc. have assembled the following data for the year ended December 31, 2016: Prepare Carlsons statement of cash flows using the indirect method. Include an accompanying...
-
Transform following Relational model into ER Model. id Author writes authorid bookid Book bookid title edition libid memberld name dob AuthorContact id contact publishes Library id bid date id...
-
A 1.100 L flask at 25 C and 1.00 atm pressure contains CO 2 (g) in contact with 100.0 mL of a saturated aqueous solution in which [CO 2 (aq)] = 3.29 x 10 -2 M. (a) What is the value of K c at 25 C...
-
The equilibrium constant for the following reaction has been estimated to be K = 10 20 at 25 C. Estimate the equilibrium concentration of NH 2 - in a solution prepared by dissolving 0.0125 mol NaNH 2...
-
Based on the information below, record the adjusting journal entries that must be made for Kisling Distributors on June 30, 20X1. The company has a June 30 fiscal year-end. Use 18 as the page number...
-
A heat exchanger produces dry steam at 100 degree Celsius from feed water at 35 degree Celsius at a rate of 2kgs^-1. the heat exchanger receives heat energy at a rate of 680 kW from the fuel used....
-
Problem 3 - Quadratic Equation Solution 0 solutions submitted (max: Unlimited) If a second order polynomial is written in the general form: then the roots (i.e. the values of x that satisfy the...
-
Ricardo has only one bank account, a chequing account into which all of his money goes, and where it remains. He has no savings account or investments. He uses a credit card for all of his purchases...
-
Why are standard costs effective in budgeting and how do we develop them?
-
A compressed air storage cylinder has a volume of 0.7m^3 and contains air at an absolute pressure of 3 MPa and temperature 30 degree celsius. A quantity of the air is released during which the...
-
1. Prepare a journal entries for each transaction/ 2. Using the journal entries as a guide, show whether each transaction would be handled as a revenue or an expense using both the accrual and cash...
-
Use the T account for Cash below to record the portion of each of the following transactions, if any that affect cash. How do these transactions affect the companys liquidity? Jan. 2 Provided...
-
On February 17, Asher Corporation acquired 3,000 shares of the 100,000 outstanding shares of Dan Co. common stock at $28.90 plus commission charges of $300. On July 11, a cash dividend of $0.95 per...
-
The following equity investment-related transactions were completed by Lance Company in 2010: Jan. 12. Purchased 1,800 shares of Baxter Company for a price of $56.50 per share plus a brokerage...
-
Plumbline Tech Corp. manufactures surveying equipment. Journalize the entries to record the following selected equity investment transactions completed by Plumbline during 2010: Feb. 2. Purchased for...
-
Finn does side work as a chef. For his most recent event, he spent six hours cooking and prepping at $50/hour. His clients provided all the raw foods and materials shown below from a list he sent...
-
Malik has an adjusted gross income (AGI) of $150,000. He donated to the local church some stock valued at $90,000 that was purchased eight months ago. His basis in this stock was $80,000. What is...
-
Seybert Systems accounts for its investment in Wang Engineering bonds as available-for-sale. Seybert's balance in accumulated other comprehensive income with respect to the Wang investment is a...

Study smarter with the SolutionInn App


