The amount of ozone, O 3 , in a mixture of gases can be determined by passing
Question:
The amount of ozone, O3, in a mixture of gases can be determined by passing the mixture through a solution of excess potassium iodide, KI. Ozone reacts with the iodide ion as follows:
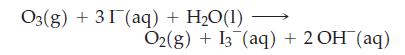
The amount of I3- produced is determined by titrating with thiosulfate ion, S2O32-:
![]()
A mixture of gases occupies a volume of 53.2 L at 18 °C and 0.993 atm. The mixture is passed slowly through a solution containing an excess of KI to ensure that all the ozone reacts. The resulting solution requires 26.2 mL of 0.1359 M Na2S2O3 to titrate to the end point. Calculate the mole fraction of ozone in the original mixture.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:





