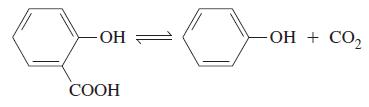
The decomposition of salicylic acid to phenol and carbon dioxide was carried out at 200.0 C, a
Question:
The decomposition of salicylic acid to phenol and carbon dioxide was carried out at 200.0 °C, a temperature at which the reactant and products are all gaseous. A 0.300 g sample of salicylic acid was introduced into a 50.0 mL reaction vessel, and equilibrium was established. The equilibrium mixture was rapidly cooled to condense salicylic acid and phenol as solids; the CO2(g) was collected over mercury and its volume was measured at 20.0 °C and 730 mmHg. In two identical experiments, the volumes of CO2(g) obtained were 48.2 and 48.5 mL, respectively. Calculate Kp for this reaction, for pressures in atmospheres.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:





