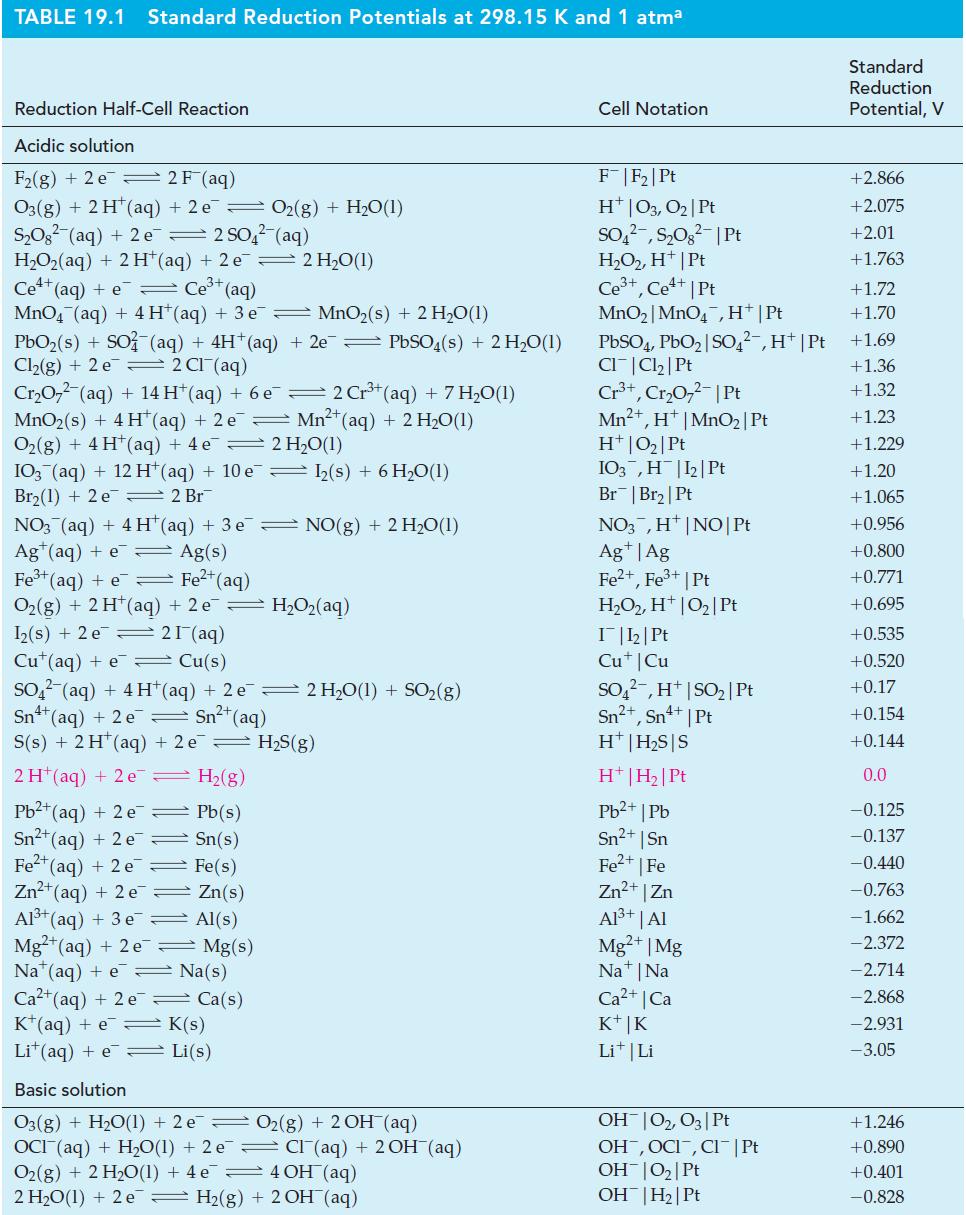
The electrode potential diagram for manganese in acidic solutions in Figure 23-14 does not include a value
Question:
The electrode potential diagram for manganese in acidic solutions in Figure 23-14 does not include a value of E° for the reduction of MnO4– to Mn2+. Use other data in the figure to establish this E°, and compare your result with the value found in Table 19.1.
Figure 23-14
![Acidic solution ([H+] = 1 M): +7 +6 MnO (purple) 0.56 V 0.56 V MnO4 (purple) -MnO42- (green) 1.70 V Basic](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1701/3/3/9/9786568634ae063e1701339977505.jpg)
Table 19.1
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:





