The equation representing the neutralization of acetic acid, CH 3 COOH, by a base B is CH
Question:
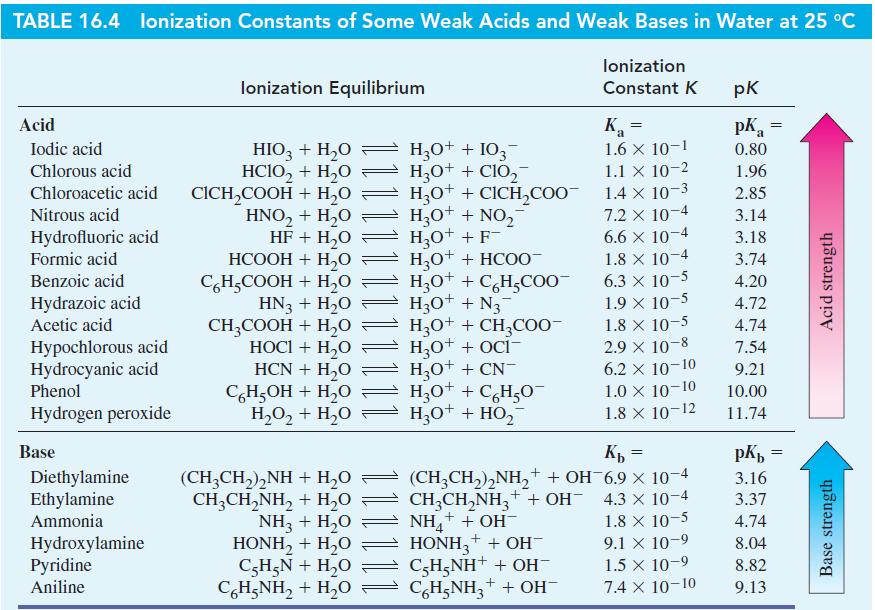
The equation representing the neutralization of acetic acid, CH3COOH, by a base B is CH3COOH(aq) + B(aq) ⇌ CH3COO-(aq) + BH+(aq). Of the bases listed in Table 16.4, which would be effective for neutralizing essentially all of the CH3COOH in a sample, assuming that CH3COOH and B are initially present in equal amounts?
Table 16.4
Transcribed Image Text:
TABLE 16.4 lonization Constants of Some Weak Acids and Weak Bases in Water at 25 °C Acid Iodic acid Chlorous acid Chloroacetic acid Nitrous acid Hydrofluoric acid Formic acid Benzoic acid Hydrazoic acid Acetic acid Hypochlorous acid Hydrocyanic acid Phenol Hydrogen peroxide Base Diethylamine Ethylamine Ammonia Hydroxylamine Pyridine Aniline lonization Equilibrium HIO3 + H₂O HClO, + H,O CICH₂COOH + H₂O HNO₂ + H₂O HF + H₂O HCOOH + H,O C6H₂COOH + H₂O HN3 + H₂O CH3COOH + H₂O HOCI + H₂O HCN + H₂O C6H5OH + H₂O H₂O₂ + H₂O (CH,CH,),NH+H,O CH,CH,NH, + H,O NH,+H,O HONH, + H,O C₂H₂N + H₂O CoH;NH, + H,O H3O+ + 103- H3O+ + CIO₂ H3O+ + CICH₂COO- H3O+ + NO₂ H₂O+ + F- H_O* + HCOO- H3O+ + C6H₂COO™ H3O+ + N3 H3O+ + CH3COO- H₂O+ + OCI- H₂O+ + CN- H3O+ + C6H₂O- H3O+ + HO₂ lonization Constant K K₁ = 1.6 X 10-1 1.1 X 10-2 1.4 x 10-3 7.2 x 10-4 6.6 x 10-4 1.8 x 10-4 6.3 x 10-5 1.9 × 10-5 1.8 x 10-5 2.9 × 10-8 6.2 X 10-10 1.0 X 10-10 1.8 × 10-12 Kb = 4.3 x 10-4 (CH3CH₂)₂NH₂+ + OH-6.9 × 10-4 CH3CH₂NH3+ + OH- NH₂+ + OH- HONH3 + + OH- C-H₂NH+ + OH- C6H5NH₂+ + OH 1.8 x 10-5 9.1 x 10-9 1.5 × 10-9 7.4 X 10-10 pk pKa 0.80 1.96 2.85 3.14 3.18 3.74 4.20 4.72 4.74 7.54 9.21 10.00 11.74 = pKb = 3.16 3.37 4.74 8.04 8.82 9.13 Acid strength Base strength
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (QA)
To neutralize all of the acetic acid in a sample we need a base t...View the full answer

Answered By

Shadrack Mulunga
I am a Biochemistry by profession. However, I have explored different fields of study. My quest to explore new fields has helped me gain new knowledge and skills in Business, clinical psychology, sociology, organizational behavior and general management, and Project Management. I count my expertise in Project management, in particular, creation of Work Break Down Structure (WBS) and use of Microsoft Project software as one of my greatest achievement in Freelancing industry. I have helped thousands of BSC and MSC students to complete their projects on time and cost-effectively using the MS Project tool. Generally, I find happiness in translating my knowledge and expertise to success of my clients. So far, i have helped thousands of students to not only complete their projects in time but also receive high grades in their respective courses. Quality and timely delivery are the two key aspects that define my work. All those who hired my services always come back for my service. If you hire my services today, you will surely return for more. Try me today!
5.00+
154+ Reviews
289+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
Don works as a financial adviser in a practice with three other advisers. They each own 25% of the business and while they each look after their own clients, they do share back office, paraplanning...
-
A base B is placed into a beaker of water with the result depicted below (water molecules have been omitted for clarity). Write the ionization reaction for this base and classify the base as being...
-
A researcher took an SRS of 4 high schools from a region with 29 high schools for a study on the prevalence of smoking among female high school students in the region. The results were as follows: a....
-
Upton Computers makes bulk purchases of small computers, stocks them in conveniently located warehouses, ships them to its chain of retail stores, and has a staff to advise customers and help them...
-
How does the effective annual rate differ between a loan requiring interest payments at maturity and another, similar loan requiring interest in advance?
-
1. Now consider the way the project was probably initiated. To what extent is the project the result of (a) An opportunity, (b) A problem, or (c) A directive? 2. Many of the system users (such as...
-
The accounting records for ADR, Inc., reflected the following amounts at the end of August, 2010: Calculate the gross profit percentage and current ratio for 2010. Cash........ $3,200 Cost of Goods...
-
A six-column table for JJW Company follows. The first two columns contain the unadjusted trial balance for the company as of July 31, 2011. The last two columns contain the adjusted trial balance as...
-
Answer the following questions for Fred corporation. Assume that the firm revenues grow at a rate of 10% per year during years 1 through 4 before leveling out at no growth for year 5 and beyond. You...
-
Write a chemical equation showing how an HCO 3 - ion can act as both an acid and a base in aqueous solution. Without doing any pH calculations, determine whether 0.10 M NaHCO 3 is acidic, basic, or...
-
With the aid of Table 16.2, predict the direction (forward or reverse) favored in each of the following acidbase reactions. Table 16.2 (a) NH4+ + OH = HO + NH3 (b) HSO4 + NO3 HNO3 + SO4- (c) CH3OH +...
-
What rules should a company use about public and private data to avoid offending employees or customers? As a group, try to write down one or two sentences of a policy on data privacy for employees...
-
You (and your team) are employed as Management Accountants working for Qantas Airways. You have been given the task of recommending a new business strategy for Qantas Airways. Question 1 1. Using...
-
Research the civil case Katz, et al. v. Panera Bread Co. and answer the following questions: Give a brief description of the lawsuit. Did Panera properly display the contents of the drink? Explain...
-
Travel Inc. sells tickets for a Caribbean cruise on ShipAway Cruise Lines to Carmel Company employees. The total cruise package price to Carmel Company employees is $70,000. Travel Inc. receives a...
-
The diagram shows four resistors R, R,r, and r that are connected to identical ideal batteries. The resistances are such that R = R >r = r. y= N Ts R ww Series Circuit V= {Rp Parallel Circuit Rank...
-
A ball falls from height of 18.5 m, hits the floor, and rebounds vertically upward to height of 15.0 m. Assume that mball -0.400 kg. (a) What is the impulse (in kg m/s) delivered to the ball by the...
-
Ben Rakusin is contemplating an expansion of his business. He believes he can increase revenues by $9,000 each month if he leases 1,500 additional square feet of showroom space. Rakusin has found the...
-
What is an access control list?
-
A diesel-powered tractor with a cost of $145,000 and estimated residual value of $7,000 is expected to have a useful operating life of 75,000 hours. During July, the generator was operated 150 hours....
-
Prior to adjustment at the end of the year, the balance in Trucks is $250,900 and the balance in Accumulated Depreciation?Trucks is $88,200. Details of the subsidiary ledger are as follows: (a)...
-
A Kubota tractor acquired on January 9 at a cost of $75,000 has an estimated useful life of 20 years. Assuming that it will have no residual value, determine the depreciation for each of the first...
-
10.2. (a) Write a MATLAB function [W,R] = house (A) that computes an implicit representation of a full QR factorization A = QR of an m x n matrix A with m>n using Householder reflections. The output...
-
What will be the output of the Expansion Permutation (in HEX format) if the input is Ox7C5DA91E. Expansion Permutation is given below: 32 1 2 3 4 | 5 4 5 6 7 8 9 8 9 10 11 12 13 12 13 14 15 16 17 16...
-
2. Show the left and right derivation for the string "aaabbabbba" using the following grammar, SaB/bA A aS/bAA/a B bS/aBB / b

Study smarter with the SolutionInn App


