The following data were obtained for compounds of nitrogen and hydrogen: (a) Show that these data are
Question:
The following data were obtained for compounds of nitrogen and hydrogen: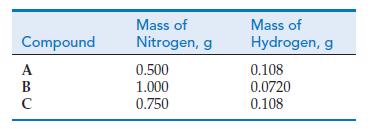
(a) Show that these data are consistent with the law of multiple proportions.
(b) If the formula of compound B is N2H2 what are the formulas of compounds A and C?
Transcribed Image Text:
Compound A B C с Mass of Nitrogen, g 0.500 1.000 0.750 Mass of Hydrogen, g 0.108 0.0720 0.108
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (3 reviews)
a To determine if the data are consistent with the law of multiple proport...View the full answer

Answered By

ALBANUS MUTUKU
If you are looking for exceptional academic and non-academic work feel free to consider my expertise and you will not regret. I have enough experience working in the freelancing industry hence the unmistakable quality service delivery
4.70+
178+ Reviews
335+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
Four capacitors, all with capacitance C, are connected in series as shown in Figure P18.50. What is the equivalent capacitance of this combination? Figure P18.50 ? C C C C
-
Why is Equation 28.5 not a full description of the relation between voltage and current in a capacitor? Vp (28.5) Xc 1/@C
-
The following data were obtained for compounds of iodine and fluorine: (a) Show that these data are consistent with the law of multiple proportions. (b) If the formula for compound A is IF, what are...
-
The functions in Exercises 1128 are all one-to-one. For each function, a. Find an equation for f -1 (x), the inverse function. b. Verify that your equation is correct by showing that f( f -1 (x)) = x...
-
Safety Auto Accessories manufactures an automobile safety seat for children that it sells through several retail chains. Safety Auto makes sales exclusively within its five-state region in the...
-
The following sample data were collected to determine the relationship between processing variables and the current gain of a transistor in the integrated circuit: Fit a regression plane and use its...
-
1. Recent consumer surveys suggest that the three most important qualities of a retail operation are price, product (quality and selection), and convenience. Based on that information, describe how...
-
Galen Stoller was killed at a railroad crossing when an AMTRAK train hit his car. The crossing was marked with a stop sign and a railroad-crossing symbol, but there were no flashing lights. Galens...
-
Sheridan Corporation manufactures wireless soundbar speakers. It is a division of Vany TV, which manufactures televisions. Sheridan sells the speakers to Vany as well as to retail stores. The...
-
There are two oxides of copper. One oxide has 20% oxygen, by mass. The second oxide has a smaller percent of oxygen than the first. What is the probable percent of oxygen in the second oxide?
-
Samples of pure carbon weighing 3.62, 5.91, and 7.07 g were burned in an excess of air. The masses of carbon dioxide obtained (the sole product in each case) were 13.26, 21.66, and 25.91 g,...
-
For each of the following systems give an example of how a mechanical engineer would have to address economic issues in its design. (a) Clothes dryer (b) Robotic lawnmower (c) Retractable stadium...
-
1. A circuit is given as shown. a) Find and label the circuit nodes b) Determine Vx V and Ix 30v www 952 2. A circuit is given as shown. www 652 852 15.2 a) Find and label the circuit nodes b)...
-
Determine the appropriate business tax of each of the following transactions/business. V if 12% vat, PT if percentage tax and E if exempt from business tax. 1. Hog dealers 2. Gasoline stations 3....
-
Matching Type 1. If a duly authorized agent acts in accordance with the orders of the principal, the latter cannot set up the _______ of the agent as to circumstance whereof he himself was, or ought...
-
3. (10 points) The following table is taken from Gary Gorton's work on financial panics: Table 2.3 U.S. National Banking Era Panics NBER Business Cycle Dates Peak-Trough September 1902-August 1904...
-
1. 40 marks Matching markets Answer the following questions. (a) 5 marks Construct a two-sided matching problem in which there is more than one stable matching. (b) 5 marks Consider a two-sided...
-
Nywening Ltd. (Nywening) operates in a highly competitive industry. Price is very important to most customers and it's very difficult for small operators such as Nywening to differentiate themselves...
-
You are planning to purchase your first home five years from today. The required down payment will be $50,000. You currently have $20,000. but you plan to contribute $500 each quarter to a special...
-
Nieland Industries had one patent recorded on its books as of January 1, 2010. This patent had a book value of $288,000 and a remaining useful life of 8 years. During 2010, Nieland incurred research...
-
Sinise Industries acquired two copyrights during 2010. One copyright related to a textbook that was developed internally at a cost of $9,900. This textbook is estimated to have a useful life of 3...
-
Karen Austin Corporation has capitalized software costs of $800,000, and sales of this product the first year totaled $420,000. Karen Austin anticipates earning $980,000 in additional future revenues...
-
Frey Company, a shoe manufacturer, has the opportunity to receive the following mixed income of cash flows over the next five years: Year-End Cash Flow 1 $400.00 2 $800.00 3 $500.00 4 $400.00 5...
-
Sonia is the Junior Accountant at East Ltd and she has recently completed the first draft of the year-end financial reports. The following financial information is extracted from the financial...
-
North Ltd is a manufacturing company. The following asset information is extracted from its property, plant, and equipment schedule on 1 July 2022: $ Plant - North Rye 1,450,000 Less Accumulated...

Study smarter with the SolutionInn App


