The following four equilibria lie to the right: (a) Rank all the acids involved in order of
Question:
The following four equilibria lie to the right:
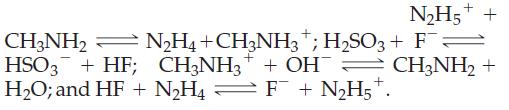
(a) Rank all the acids involved in order of decreasing acid strength.
(b) Rank all the bases involved in order of decreasing base strength.
(c) State whether each of the following two equilibria lies primarily to the right or to the left:
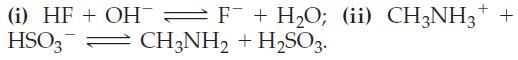
Transcribed Image Text:
N₂H5+ + CH3NH₂ N₂H4+CH3NH3 + H₂SO3 + F + → HSO3 + HF; CH3NH3 + OH H₂O; and HF + N₂H4 = F¯ + N₂H5+. CH3NH₂ +
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (2 reviews)
a Ranking acids by decreasing acid strength The strength of an acid is determined by its tendency to ...View the full answer

Answered By

Lamya S
Highly creative, resourceful and dedicated High School Teacher with a good fluency in English (IELTS- 7.5 band scorer) and an excellent record of successful classroom presentations.
I have more than 2 years experience in tutoring students especially by using my note making strategies.
Especially adept at teaching methods of business functions and management through a positive, and flexible teaching style with the willingness to work beyond the call of duty.
Committed to ongoing professional development and spreading the knowledge within myself to the blooming ones to make them fly with a colorful wing of future.
I do always believe that more than being a teacher who teaches students subjects,...i rather want to be a teacher who wants to teach students how to love learning..
Subjects i handle :
Business studies
Management studies
Operations Management
Organisational Behaviour
Change Management
Research Methodology
Strategy Management
Economics
Human Resource Management
Performance Management
Training
International Business
Business Ethics
Business Communication
Things you can expect from me :
- A clear cut answer
- A detailed conceptual way of explanation
- Simplified answer form of complex topics
- Diagrams and examples filled answers
4.90+
46+ Reviews
54+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
Rank the following compounds in order of decreasing acid strength using periodic trends. Strongest ---> weakest BH3 HBr H2S H2Se
-
Rank the following compounds in order of decreasing acid strength: CH2OH COOH
-
The Crazy Eddie fraud may appear smaller and gentler than the massive billion-dollar frauds exposed in recent times, such as Bernie Madoffs Ponzi scheme, frauds in the subprime mortgage market, the...
-
Built-Tite uses job order costing. The T-account below summarizes Factory overhead activity for the current year. Factory Overhead Debit Credit 16,200 106,600 25,200 60,200 1. Compute total applied...
-
Cleveland Corporation is interested in acquiring Lewis Tool Company by swapping 0.4 share of its stock for each share of Lewis stock. Certain financial data on these companies are given in the...
-
Refer to the data in Problem 6-21. Required: Repeat the requirements in Problem 6-21 using the FIFO method.
-
True or False: Multiple roots can exist when using IRR and MIRR methods.
-
Starr Co. had sales revenue of $540,000 in 2012. Other items recorded during the year were: Cost of goods sold $330,000 Salaries and wages expense 120,000 Income tax expense 25,000 Increase in value...
-
Fickle Sickles collects 15,000 checks per 365-day year with average amount $170 and total delay 5 days. A lockbox system would reduce that delay to 3 days, and it would also reduce FISI's check...
-
Given that K a for HI is about 10 9 , estimate the equilibrium concentrations of HI and I - in (a) 1.0 M HI(aq); (b) 1.0 M NaI(aq).
-
An aqueous solution of two weak acids has a stoichiometric concentration, c, in each acid. If one acid has a K a value twice as large as the other, show that the pH of the solution is given by the...
-
Given the limited research support for the MBTI, what are the concerns regarding organizations continuing to use it?
-
Melanie files single. Last year, she received $11,500 in social security benefits and had $1,500 in interest income. She had no other income or adjustments. Melanie's modified AGI for determining...
-
A boy pushes on a 4.50-kg sled with a force of 10.0 N at 52.0 angle with respect to the horizontal surface of a frozen pond. The sled moves with constant velocity. What is the normal force acting on...
-
What are the implications of ethical leadership for corporate governance, corporate social responsibility, and sustainable business practices in an increasingly interconnected and socially conscious...
-
When Maggie applies the brakes of her car, the car slows uniformly from 15.0 m/s to a complete stop in 2.55. How many meters before the stop sign must she apply her brakes in order to stop at the...
-
A car with an initial speed of 4.3 m/s accelerates uniformly at the rate of 3.0 m/s? for 5 seconds. a. Find the final speed? b. Find the distance traveled.
-
Willco Inc. manufactures electronic parts. They are analyzing their monthly maintenance costs to determine the best way to budget these costs in the future. They have collected the following data for...
-
The vapor pressure of the liquid NH, is measured at different temperatures. The following vapor pressure data are obtained. Temperature, K P, mmHg 217.1 223.4 234.7 588.1 Calculate the enthalpy of...
-
Journalize the following transactions in the accounts of Laser Tech Co., a medical equipment company that uses the direct write-off method of accounting for uncollectible receivables: Feb. 23. Sold...
-
Journalize the following transactions in the accounts of Food Unlimited Company, a restaurant supply company that uses the allowance method of accounting for uncollectible receivables: Jan. 18. Sold...
-
Tech Savvy, a computer consulting firm, has decided to write off the $8,375 balance of an account owed by a customer, Nick Wadle. Journalize the entry to record the write-off, assuming that (b) The...
-
Simplify the following square root xpression. (-18) (-2)
-
Under its executive stock option plan, National Corporation granted 30 million options on January 1, 2021, that permit executives to purchase 30 million of the company's $1 par common shares within...
-
Calculate the determinant of the matrix A, where A -3 1 51 -60 3 41

Study smarter with the SolutionInn App


