The following very strong acids are formed by the reactions indicated: (a) Identify the Lewis acids and
Question:
The following very strong acids are formed by the reactions indicated:
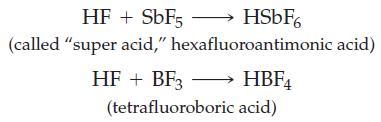
(a) Identify the Lewis acids and bases.
(b) To which atom is the H atom bonded in each acid?
Transcribed Image Text:
HF + SbF5- (called "super acid," HSbF6 hexafluoroantimonic acid) HF + BF3 →→→→ HBF4 (tetrafluoroboric acid)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (4 reviews)
a Identify the Lewis acids and bases HF SbF5 HSbF6 In this reactionHF is the Lew...View the full answer

Answered By

PALASH JHANWAR
I am a Chartered Accountant with AIR 45 in CA - IPCC. I am a Merit Holder ( B.Com ). The following is my educational details.
PLEASE ACCESS MY RESUME FROM THE FOLLOWING LINK: https://drive.google.com/file/d/1hYR1uch-ff6MRC_cDB07K6VqY9kQ3SFL/view?usp=sharing
3.80+
3+ Reviews
10+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
Use the concept of hybrid orbitals to describe the bonding in the strong acids given in Exercise 78. Exercise 78 The following very strong acids are formed by the reactions indicated: (a) Identify...
-
An investor wishes to analyse the effects of different compounding frequencies Suppose 1000 is invested for 1 year at an interest rate of 5 per annum compounded Assume there are 365 days in 1 year
-
In the following acid-base reactions, 1. Determine which species are acting as electrophiles (acids) and which are acting as nucleophiles (bases). 2. Use the curved-arrow formalism to show the...
-
For what number does the principal square root exceed eight times the number by the largest amount?
-
Briefly describe each of the following takeover defenses against a hostile merger: (a) White knight, (b) Poison pill, (c) Greenmail, (d) Leveraged recapitalization, (e) Golden parachutes, and (f)...
-
GianAuto Corporation manufactures automobiles, vans, and trucks. Among the various GianAuto plants around the United States is the Denver cover plant, where vinyl covers and upholstery fabric are...
-
True or False: If \(E R R>M A R R\), then \(I R R>E R R>M A R R\).
-
The following trial balance of Oakley Co. does not balance. Each of the listed accounts should have a normal balance per the general ledger. An examination of the ledger and journal reveals the...
-
Two years after the first round XMP GmbH receives a second offer form B-Capital. B-Capital offers to invest 12,500,000.00 at a 60,000,000.00 pre-money valuation. What is the founders' post-round...
-
The molecular solid I 2 (s) is only slightly soluble in water but will dissolve to a much greater extent in an aqueous solution of KI, because the I 3 - anion forms. Write an equation for the...
-
CO 2 (g) can be removed from confined quarters (such as a spacecraft) by allowing it to react with an alkali metal hydroxide. Show that this is a Lewis acidbase reaction. For example, CO(g) + LiOH(s)...
-
The null and alternate hypotheses are: H 0 : 0 H 1 : > 0 The following sample information shows the number of defective units produced on the day shift and the afternoon shift for a sample of...
-
Ethan is 0.73 meters tall standing 9.05 meters away from Sreekanth who is 2.2 meters tall. If Ethan looks at Sreekanth's head, what is the angle, in degrees, of elevation of Ethan's eyes?
-
An electromagnetic wave is radiated from an antenna with a frequency of 50.0 kHz. This electromagnetic wave has an average intensity of 76.5 MW/m. (a) What is the wave's maximum magnetic field...
-
An electric wire carries a dc current of 40 A vertically upward. (a) What is the magnetic field due to this current at a point P, 10 cm due north of the wire ? (b) What is its direction?
-
One vector has coordinates (1.58, -9.78). A second vector has coordinates (-9.16, -3.8). What is the magnitude of their sum?
-
One vector has magnitude 8 and is at an angle 33. A second vector has magnitude 9 and is at an angle 357. What is the x component of their sum?
-
You have bought 100 $1,000 10-year government bonds that pay a coupon rate of 10% p.a. (semi-annual compounding). If the market yield is 18% p.a. compounding semi-annually, how much did you spend?
-
5. How much would you need to deposit in an account now in order to have $5,000 in the account in 5 years? Assume the account earns 2% interest compounded monthly. 10. You deposit $300 each month...
-
Determine the income participation of Hassell and Lawson, according to each of the five assumptions as to income division listed in Exercise 12-3 if the years net income is $380,000.
-
Casey Fisher and Logan Baylor formed a partnership in which the partnership agreement provided for salary allowances of $40,000 and $35,000, respectively. Determine the division of a $20,000 net loss...
-
Ben Bowman and Savannah Mapes formed a limited liability company with an operating agreement that provided a salary allowance of $75,000 and $60,000 to each member, respectively. In addition, the...
-
Accounting standards are the technical process of balancing accounts. rules for preparing financial statements. the levels of tax payments needed. the rules for performing an audit
-
Carol buys a couch from Company X. Company X retains a security interest in the couch until Carol pays off the balance of the purchase price. In this scenario, Company X is the
-
You buy a new Ford F150. The tires that come with the truck are final goods. intermediate goods. transfer goods. financial goods

Study smarter with the SolutionInn App


