The initial rate of reaction (20.3) is found to be 1.7 x 10 -3 M s -1
Question:
The initial rate of reaction (20.3) is found to be 1.7 x 10-3 M s-1. Assume that this rate holds for 2 minutes. Start with 175 mL of 1.55 M H2O2(aq) at t = 0. How many milliliters of O2(g), measured at 24°C and 757 mmHg, are released from solution in the first minute of the reaction?
Reaction (20.3)
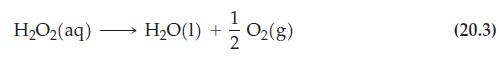
Transcribed Image Text:
H,Oz(aq) H₂O(1) + 1/02 (8) (20.3)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 33% (3 reviews)
ANSWER To determine the amount of O2 released in the first minute of the reaction we need to use the ...View the full answer

Answered By

Utsab mitra
I have the expertise to deliver these subjects to college and higher-level students. The services would involve only solving assignments, homework help, and others.
I have experience in delivering these subjects for the last 6 years on a freelancing basis in different companies around the globe. I am CMA certified and CGMA UK. I have professional experience of 18 years in the industry involved in the manufacturing company and IT implementation experience of over 12 years.
I have delivered this help to students effortlessly, which is essential to give the students a good grade in their studies.
3.50+
2+ Reviews
10+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
Googles ease of use and superior search results have propelled the search engine to its num- ber one status, ousting the early dominance of competitors such as WebCrawler and Infos- eek. Even later...
-
For the reaction I (aq) + OCl (aq) OI (aq) + Cl (aq) occurring in aqueous solution, the following mechanism has been proposed: a. Derive the rate law expression for this reaction based on this...
-
Eva pays $3,000 per month to rent her house. She has a garage but is considering turning the space into a hair styling studio. She expects to earn $3,000 a month from this new business. Instead, she...
-
Predict your results. Write "yes" or "no," depending on whether you think the plate will show growth. Give the reason (s) for your predictions. Observe the colonies through the petri plate lids. Do...
-
Describe the component approach to computing depreciation.
-
On January 4, 2010, the Franc Company purchased for $27,000 a patent that had been filed eight years earlier. The patent covers a manufacturing process that the company plans to use for 15 years. On...
-
State the appropriate null and alternate hypotheses for determining whether to conclude that the failure rates differ among the four lines. Exercises 49 refer to the following data: Electric motors...
-
What is block ownership? How does it affect corporate governance?
-
Determine the annual tax effects (increase or decrease to taxes paid) if they decide to purchase the new machine. Assume a tax rate of 30% Depreciate assets based on the information in the case (do...
-
We have seen that the units of k depends on the overall order of a reaction. Derive a general expression for the units of k for a reaction of any overall order, based on the order of the reaction (o)...
-
Use the method of Exercise 75 to determine the volume of 0.1000 M KMnO 4 required to titrate 5.00 mL samples of H 2 O 2 (aq) for each of the entries in Table 20.1. Plot these volumes of KMnO 4 (aq)...
-
In an attempt to prepare propylbenzene, a chemist alkylated benzene with 1-chloropropane and aluminum chloride. However, two isomeric hydrocarbons were obtained in a ratio of 2:1, the desired...
-
A 35-year-old person who wants to retire at age 65 starts a yearly retirement contribution in the amount of $5,000. The retirement account is forecasted to average a 6.5% annual rate of return,...
-
November 1 of the current year, a company entered into a purchase contract (not subject to revision or cancellation) to purchase 900 units of inventory for $50 per unit before January 31 of the...
-
In the context of organizational culture, how do underlying assumptions and beliefs shape employee behavior, and what methods can be used to align these with organizational goals ?
-
Monthly payments of $300 are made at the beginning of each month on a lease having a book value of $15,050. What is the term of the lease if the lessee's cost of borrowing is 12% compounded monthly?
-
An item is purchased for 24 monthly payments of $150. The first payment is due on the date of sale, and the interest rate charged on the balance is 18% compounded monthly. What is the purchase price...
-
Suppose American Airlines acquired a new Boeing 747 airplane for $250 million. Its expected residual value was $70 million. The companys annual report indicated that straight-line depreciation was...
-
Consider model (9.18). What is the effect on the model parameter estimates, their standard errors, and the goodness-of-fit statistics when (a) The times at risk are doubled, but the numbers of deaths...
-
What is the normal balance for each of the following accounts? (a) Accounts Receivable. (b) Cash. (c) Owners Drawing. (d) Accounts Payable. (e) Service Revenue. (f) Salaries Expense. (g) Owners...
-
Indicate whether each of the following accounts is an asset, a liability, or an owners equity account and whether it has a normal debit or credit balance: (a) Accounts Receivable, (b) Accounts...
-
For the following transactions, indicate the account debited and the account credited. (a) Supplies are purchased on account. (b) Cash is received on signing a note payable. (c) Employees are paid...
-
How does phenotypic plasticity enable organisms to adapt to fluctuating environmental conditions, and what are the molecular and genetic mechanisms that underlie this plasticity ?
-
Can you elaborate on the concept of canalization and its role in buffering phenotypic traits against genetic and environmental perturbations, as well as its implications for evolutionary robustness ?
-
How do epigenetic modifications contribute to the regulation of phenotypic traits, and what is the role of transgenerational epigenetic inheritance in the continuity and divergence of phenotypes...

Study smarter with the SolutionInn App


