The ionization energies of Li, Be + , B 2+ , and C 3+ are, respectively, 520,
Question:
The ionization energies of Li, Be+, B2+, and C3+ are, respectively, 520, 1757, 3659, and 6221 kJ mol–1. The ionization energies Na, Mg+, Al2+, and Si3+ are (from Table 9.4) 495.8, 1451, 2745, and 4356 kJ mol–1. Plot a graph of the square roots of the ionization energies versus the nuclear charge for these two series. Explain the observed relationship with the aid of Bohr’s expression for the binding energy of an electron in a one-electron atom.
Table 9.4
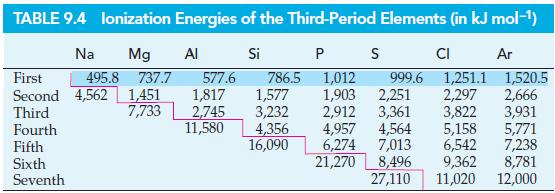
Transcribed Image Text:
TABLE 9.4 lonization Energies of the Third-Period Elements (in kJ mol-¹) Si P S Ar Na Mg Al 495.8 737.7 First 577.6 1,012 999.6 1,251.1 1,520.5 Second 4,562 1,451 1,817 1,903 2,251 2,297 2,666 Third 7,733 2,745 2,912 3,361 3,822 3,931 Fourth 4,957 4,564 5,158 5,771 6,274 7,013 6,542 7,238 21,270 8,496 9,362 8,781 27,110 11,020 12,000 Fifth Sixth Seventh 786.5 1,577 3,232 11,580 4,356 16,090 CI
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (3 reviews)
Plotting the data Firstlets calculate the square roots of the ionization energies Ion Ionization energy kJ mol ionization energy Li 520 2280 Be 1757 4...View the full answer

Answered By

Somshukla Chakraborty
I have a teaching experience of more than 4 years by now in diverse subjects like History,Geography,Political Science,Sociology,Business Enterprise,Economics,Environmental Management etc.I teach students from classes 9-12 and undergraduate students.I boards I handle are IB,IGCSE, state boards,ICSE, CBSE.I am passionate about teaching.Full satisfaction of the students is my main goal.
I have completed my graduation and master's in history from Jadavpur University Kolkata,India in 2012 and I have completed my B.Ed from the same University in 2013. I have taught in a reputed school of Kolkata (subjects-History,Geography,Civics,Political Science) from 2014-2016.I worked as a guest lecturer of history in a college of Kolkata for 2 years teaching students of 1st ,2nd and 3rd year. I taught Ancient and Modern Indian history there.I have taught in another school in Mohali,Punjab teaching students from classes 9-12.Presently I am working as an online tutor with concept tutors,Bangalore,India(Carve Niche Pvt.Ltd.) for the last 1year and also have been appointed as an online history tutor by Course Hero(California,U.S) and Vidyalai.com(Chennai,India).
4.00+
2+ Reviews
10+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
The ionization energies of sodium (in kJ/mol), starting with the first and ending with the eleventh, are 495.9, 4560, 6900, 9540, 13,400, 16,600, 20,120, 25,490, 28,930, 141,360, 170,000. Plot the...
-
The ionization energies of the first few polyacenes are 9.4 eV for benzene, 8.3 eV for naphthalene, 7.6 eV for anthracene, and 7.0 eV for tetracene. Use these data to calculate values of and in the...
-
The ionization energy of O2 is smaller than the ionization energy of atomic O; the opposite is true for the ionization energies of N2 and atomic N. Explain this behavior in terms of the molecular...
-
Equation 11.14 can be expressed in "coordinate-free" form by writing P0 cos = P0 r. Do so, and likewise for Eqs. 11.17, 11.18. 11.19, and 11.21.
-
Cost allocation bases are ideally based on a cause-and-effect basis, but they are used to allocate fixed overhead. Is this inconsistent?
-
Stephen Wernet has claimed that resources are the crucial link between operation and survival and that organizations change only when they experience a significant shift in resources. Do you agree or...
-
What graphical tool enables us to delineate what is within the scope of a system, product, or services boundaries?
-
Internet CaseCalPFRS. While the examples in this chapter have focused on a single-employer plan, many states operate statewide plans, referred to as Public Employee Retirement Systems (PERS), to...
-
Marigold Hills Ltd. issued five-year bonds with a face value of $160,000 on January 1. The bonds have a coupon interest rate of 7% and interest is paid semi-annually on June 30 and December 31. The...
-
The B. Hall Real Estate Investment Corporation has identified four small apartment buildings in which it would like to invest. Mrs. Hall has approached three savings and loan companies regarding...
-
Elements 114116 have recently been reported to be synthesized. Using data given below and the periodic law, fill in the missing data for these elements. The entries for each element are organized as...
-
Evaluate Gregs decision using the guidelines for ethical decision making in Chapter 2. Assuming that Greg decides not to support Micheles current behaviour and chooses to confront in a helpful...
-
In P shown at the right, QPR RPS. Find the indicated measure. mQR
-
Complete a method named findValue which accepts an IntArray and an Int value and returns whether the array contains the passed value. Your method should not modify the passed array. However, you...
-
java code ReadFile Create-a linked list from an input file (input.txt) that contains an even number of first names. The number of items in the file is unknown. Split Create-a split function that...
-
answer this question in Java and only if you are fully confident of the answer. The code should work without any errors. These are not stocks, but transactions that have occurred. Cashback value can...
-
How would I go about making a dequeue method given that we have _front _rear and _size variables as well as a linkedList to stare it in. assuming all test cases should pass public T dequeue() { //to...
-
Inheritance Diagrams Draw diagrams that show inheritance for each of the class groups below. Use Rectangles with names for classes and arrows to show inheritance. I recommend using Lucidchartfor...
-
a. Boeing (BA) (25%), Caterpillar (CAT) (25%), Chevron (CVX) (25%), Coca-Cola (KO) (25%) b. BA (10%), CAT (20%), CVX (20%), KO (50%) c. BA (15%), CAT (55%), CVX (15%), KO (15%) d. Which portfolio...
-
Describe a job you have had in the past or a job you are very familiar with. Indicate the negative aspects of the job and how it could be improved with current human resource management techniques.
-
Is it possible for cost-such as salaries or depreciation to end up as assets on the balance sheet? Explain.
-
What is meant by the term cost behavior?
-
A variable cost is a cost that varies per unit of product, whereas a fixed cost is constant per unit of product. Do you agree? Explain.
-
Can you help me understand and elaborate about consumer protection laws in Qatar. from this links, you can add more link resources. Thanks! Links: 1....
-
Two muscles at the back of your leg, called lateral and medial heads of the gastrocnemius muscle, exert forces L and M respectively, on the Achilles tendon (Figure 3-31). The forces are both 516 N...
-
Treasury bond prices Bond Principal ($) Time to Maturity (yrs) Annual Coupon ($)* Bond Price ($) 100 0.5 0.0 98 100 1.0 0.0 95 100 1.5 6.2 101 100 2.0 8.0 102 *Half the stated coupon is paid every...

Study smarter with the SolutionInn App


