The kinetics of the decomposition of phosphine at 950 K was studied by injecting PH 3 (g)
Question:
The kinetics of the decomposition of phosphine at 950 K
![]()
was studied by injecting PH3(g) into a reaction vessel and measuring the total pressure at constant volume.
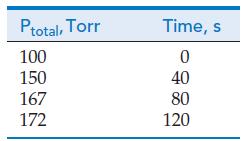
What is the rate constant of this reaction?
Transcribed Image Text:
4 PH3(g) P4(g) + 6H₂(g)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 0% (2 reviews)
The kinetics of the decomposition of phosphine at 950 K was studied by heating h...View the full answer

Answered By

Nyron Beeput
I am an active educator and professional tutor with substantial experience in Biology and General Science. The past two years I have been tutoring online intensively with high school and college students. I have been teaching for four years and this experience has helped me to hone skills such as patience, dedication and flexibility. I work at the pace of my students and ensure that they understand.
My method of using real life examples that my students can relate to has helped them grasp concepts more readily. I also help students learn how to apply their knowledge and they appreciate that very much.
4.00+
1+ Reviews
10+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
The rate of the reaction was studied by injecting CO(g) into a reaction vessel and measuring the total pressure at constant volume. What is the rate constant of this reaction? 2 CO(g) CO(g) + C(s)
-
In many experimental petrology labs, argon is used to pressurize reaction vessels. The argon comes in cylinders 1.55 m long, with an internal diameter of 11 cm. The argon pressure is 2200 psi at 25C....
-
The rate law for the decomposition of phosphine (PH3) is It takes 120 s for the concentration of 1.00 M PH3 to decrease to 0.250 M. How much time is required for 2.00 M PH3 to decrease to a...
-
Consider the following hypotheses: H 0 : = 140 H 1 : 140 Given that x = 148.1, s = 37.5, n = 20, and = 0.02, answer the following questions: a. What conclusion should be drawn? b. Use PHStat to...
-
What two unusual accounting actions are taken when a long-term operating asset is classified as held for sale?
-
List the situations that warrant use of JAD in place of personal organizational interviews.
-
The payroll disbursements were for two persons named Ciotty and Ciotti with the same first name and address. The interesting observation is that Ciotty is dated February 28, 2019, or after while...
-
Reddy Industries has the following patents on its December 31, 2011, balance sheet. The following events occurred during the year ended December 31, 2012.1. Research and development costs of $245,700...
-
Unusual shapes! At this point, we've gotten pretty comfortable with calculating the perimeter and area of squares and rectangles. In this assignment, we'll make a program that calculates these values...
-
The rate of an enzyme-catalyzed reaction can be slowed down by the presence of an inhibitor (I) that reacts with the enzyme in a rapid equilibrium process. By adding this step to the mechanism for...
-
The reaction A + B products is first order in A, first order in B, and second order overall. Consider that the starting concentrations of the reactants are [A] 0 and [B] 0 , and that x represents...
-
Indiana Electronic Workers Pension Trust Fund (IBEW) is a retirement system that provides benefits to electrical workers in Indiana. IBEW is also a stockholder of Wal-Mart corporation. Wal-Mart de...
-
Examine the four sets of emergent strategy theories. Of the four theories, which is likely to help address conflict in a change management process? Explain your answer.
-
Bond J has a coupon rate of 3 percent. Bond K has a coupon rate of 9 percent. Both bonds have 18 years to maturity, make semiannual payments, and have a YTM of 6 percent. If interest rates suddenly...
-
After reading the case, please provide your opinion about whether the ChevronDefense should continue to apply today. Are there circumstances under which the rule should not apply? ...
-
Look for and post an article regarding the challenges faced with Integrated Supply Chains. Choose one issue from the article to discuss and troubleshoot. How did the company mitigate and solve this...
-
If you own a home worth $185,000 and your unpaid mortgage is $150,000. What is your equity in the home?
-
Selkirk Company obtained a $15,000 note receivable from a customer on January 1, 2011. The note, along with interest at 10%, is due on July 1, 2011. On February 28, 2011, Selkirk discounted the note...
-
If M = 7, s = 2, and X = 9.5, what is z?
-
Selected worksheet data for Nicholson Company are presented below. Instructions(a) Fill in the missing amounts.(b) Prepare the adjusting entries that weremade. Adjusted Trial Balance Account Titles...
-
Emil Skoda Company had the following adjusted trial balance. Instructions(a) Prepare closing entries at June 30, 2010.(b) Prepare a post-closing trialbalance. EMIL SKODA COMPANY Adjusted Trial...
-
Apachi Company ended its fiscal year on July 31, 2010. The company??s adjusted trial balance as of the end of its fiscal year is as shown at the top of page 182. Instructions(a) Prepare the closing...
-
3. [7 points; 3, 1, 1, 2 points respectively] In the last couple of years Wells Fargo, one of the largest banks in the U.S., has been accused of bank scandals. [Hint: Internet research is...
-
13. a) Luna Corporation has a beta of 1.5, 10 billion in equity, and 5 billion in debt with an interest rate of 4%. Assume a risk-free rate of 0.5% and a market risk premium of 6%. Calculate the WACC...
-
14. a) Explain what is meant by the Fisher Separation Theorem (FST). Graphically demonstrate FST for the case where an individual ends up lending in financial markets. (40 marks) b) Graphically...

Study smarter with the SolutionInn App


