The solubility of Cl 2 (g) in water is 6.4 g L 1 at 25 C. Some
Question:
The solubility of Cl2(g) in water is 6.4 g L–1 at 25 °C. Some of this chlorine is present as Cl2, and some is found as HOCl or Cl–. For the hydrolysis reaction
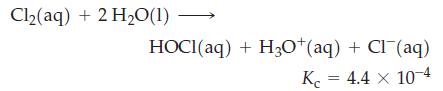
For a saturated solution of Cl2 in water, calculate [Cl2], [HOCl], [H3O+], and [Cl–].
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:





