The standard enthalpy of formation of gaseous H 2 O at 298.15 K is -241.82 kJ mol
Question:
The standard enthalpy of formation of gaseous H2O at 298.15 K is -241.82 kJ mol-1. Using the ideas contained in Figure 7-16, estimate its value at 100.0 °C given the following values of the molar heat capacities at constant pressure: H2O(g): 33.58 J K-1 mol-1; H2(g): 28.84 J K-1 mol-1; O2(g): 29.37 J K-1 mol-1. Assume the heat capacities are independent of temperature.
Figure 7-16
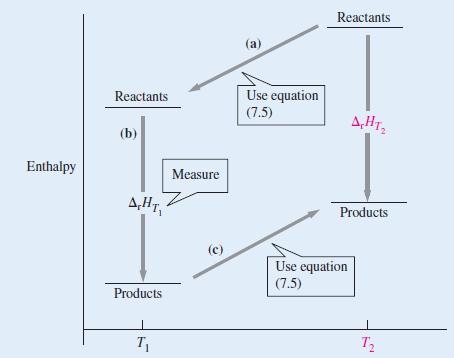
Transcribed Image Text:
Enthalpy Reactants (b) A.HT, Products T₁ Measure (c) (a) Use equation (7.5) Reactants A,HT₂2 Products Use equation (7.5) T₂
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (3 reviews)
To estimate the standard enthalpy of formation of gaseous H2O at 1000 C 37315 K using the ideas cont...View the full answer

Answered By

Nithesh M
This is Vijaya Chandra, I was graduated from JNTU Engineering College in Mechanical Engineering. I have always shown a keen interest in physics, which is evident from his exceptional academic record in the subject. During my academic years, I was excelled in physics, consistently achieving high grades and performing well in exams. In addition to academic background, I worked as a tutor for 5 years in local village by helping students of all ages and skill levels achieve their goals. As a tutor, I have taught students of all classes for a period of five years, including graduate-level physics and have a deep understanding of the subject and passion for teaching has allowed me to develop unique teaching methods that make complex concepts easy to understand. I am comfortable working with students one-on-one or in small groups, and I am dedicated to helping them build confidence and succeed in their studies. Outside tutoring, I enjoyed playing cricket and surfing internet browser.
0.00
0 Reviews
10+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
The molar heat capacity of ethane is represented in the temperature range 298 K to 400 K by the empirical expression Cp,m/ (J K-1 mol-1) = 14.73 + 0.1272(T/K). The corresponding expressions for C(s)...
-
The standard enthalpy of formation of the metallocene bis (benzene) chromium was measured in a calorimeter. It was found for the reaction Cr (C6H6)2(s) Cr(s) + 2 C6H6 (g) that Uo (583 K) = +8.0 kJ...
-
The head of a strike anywhere match contains tetraphosphorus trisulfide, P4S3. In an experiment, a student burned this compound in an excess of oxygen and found that it evolved 3651 kJ of heat per...
-
Secondary xylem and phloem in dicot stem are produced by (a) Phellogen (b) Apical meristems (c) Axillary meristems (d) Vascular cambium
-
During the current month, a company that uses a job order cost accounting system incurred a monthly factory payroll of $75,000, paid in cash. Of this amount, $29,000 is classified as indirect labor...
-
A bar of \(20 \mathrm{~mm}\) diameter is subjected to a pull of \(30 \mathrm{kN}\). The measured extensions over a guage length of \(200 \mathrm{~mm}\) is \(0.1 \mathrm{~mm}\) and the change in...
-
Which System/Product Life Cycle Phases may not be covered by the ConOps and why?
-
On December 3, Ainge Printing purchased inventory listed at $7,400 from Craig Paper Supply. Terms of the purchase were 3/10, n/20. Ainge Printing also purchased inventory from Tippetts Ink Wholesale...
-
Research: What is a sovereign nation, how many sovereign nations are recognized as members by the United Nations and how many sovereign nations are recognized by the US State Department. Compare the...
-
How might one employee coach or train another employee by use of Twitter and text messaging?
-
How much heat is required to convert 10.0 g of ice at -5.0 C to steam at 100.0 C? The temperature dependent constant-pressure specific heat capacity of ice is c p (T)/(kJ kg -1 K -1 ) = 1.0187T -...
-
The oxidation of NH 3 (g) to NO(g) in the Ostwald process must be very carefully controlled in terms of temperature, pressure, and contact time with the catalyst. This is because the oxidation of NH...
-
1. What barriers to effective communication existed in Aluminum Elements Corp.? 2. How did author deal with these? 3. What would you do differently? 4. Identify and discuss reasons why John was upset...
-
Jeff purchased a card for $180 that gives him 20 visits to a new gym and includes a one time fee for unlimited use of the sauna. After 5 visits, he has $123.75 left on the card, and after 11 visits,...
-
A study examined transformer voltage sags and swells. For a sample of 106 transformers built for heavy industry, the mean number of sags per week was 40 and the mean number of swells per week was...
-
The temperature of a 50 g sample of aluminum is raised from 20C to 60C when 440 cal of heat are added. The specific heat capacity of the aluminum will be?
-
Provide a minimum of a 3-year history of the following: unemployment rate in the United States, inflation rate in the United States, and labor force participation rate in the United States. Indicate...
-
The following graph shows the market for euros, which is initially in equilibrium. Suppose an economic expansion in Canada leads to an increase in the incomes of Canadian households, causing imports...
-
Consider the valve opening data displayed in Table 11.1. Suppose the data represent valves produced on four different machines on three different shifts and that the quality controllers want to know...
-
Using a graphing utility, graph y = cot -1 x.
-
Gross Profit Method Presented below is information related to Jerrold Corporation for the current year. Compute the ending inventory , assuming that (a) Gross profit is 40% of sales; (b) Gross profit...
-
Retail Inventory Method Presented below is information related to McKenna Company. (a) Compute the ending inventory at retail. (b) Compute a cost-to-retail percentage (round to two decimals) under...
-
Retail Inventory Method Presented below is information related to Kuchinsky Company. Compute the inventory by the conventional retail inventory method. Retail Cost Beginning inventory Purchases...
-
Determine whether each relation is a function. Explain why or why not. 1. {(3,2), (-1, 3), (5, 2)} 2. {(9,-1), (3, 6), (-2, 4), (3,0)} Determine if each equation defines y as a function of x. Explain...
-
How does organizational learning differ from individual learning, and what mechanisms can be implemented to foster a culture of continuous improvement and knowledge sharing ?
-
The Kirkland Department of Cheyenne Company began the month of December with work in process inventory of 3,520 units that are 100% complete as to materials and 20% complete as to conversion costs....

Study smarter with the SolutionInn App


