The standard enthalpy of formation of NH 3 (g) is -46.11 kJ/mol. What is r H
Question:
The standard enthalpy of formation of NH3(g) is -46.11 kJ/mol. What is ΔrH° for the following reaction?
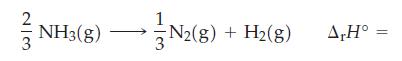
Transcribed Image Text:
2 NH3(g) -N2(g) + H₂(g) N2(8) A,Hº
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (2 reviews)
The reaction given is NH3 9 N2g Hg The enthalpy change of reaction AH is calculated using the ...View the full answer

Answered By

Aysha Ali
my name is ayesha ali. i have done my matriculation in science topics with a+ . then i got admission in the field of computer science and technology in punjab college, lahore. i have passed my final examination of college with a+ also. after that, i got admission in the biggest university of pakistan which is university of the punjab. i am studying business and information technology in my university. i always stand first in my class. i am very brilliant client. my experts always appreciate my work. my projects are very popular in my university because i always complete my work with extreme devotion. i have a great knowledge about all major science topics. science topics always remain my favorite topics. i am also a home expert. i teach many clients at my home ranging from pre-school level to university level. my clients always show excellent result. i am expert in writing essays, reports, speeches, researches and all type of projects. i also have a vast knowledge about business, marketing, cost accounting and finance. i am also expert in making presentations on powerpoint and microsoft word. if you need any sort of help in any topic, please dont hesitate to consult with me. i will provide you the best work at a very reasonable price. i am quality oriented and i have 5 year experience in the following field.
matriculation in science topics; inter in computer science; bachelors in business and information technology
_embed src=http://www.clocklink.com/clocks/0018-orange.swf?timezone=usa_albany& width=200 height=200 wmode=transparent type=application/x-shockwave-flash_
4.40+
11+ Reviews
14+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
Jeremiah Jones is going off to college and he signed a lease with Apartments Are Us. The apartment was willing to rent the apartment to Jeremiah as long as he had a guarantor Jeremiah's mom signed as...
-
The standard enthalpy of formation of the metallocene bis (benzene) chromium was measured in a calorimeter. It was found for the reaction Cr (C6H6)2(s) Cr(s) + 2 C6H6 (g) that Uo (583 K) = +8.0 kJ...
-
At 25°C, the standard enthalpy of formation of HF(aq) is given by -320.1 kJ/mol; of OH-2(aq), it is -229.6 kJ/mol; of F-(aq), it is -329.1 kJ/mol; and of H2O(l), it is -285.8 kJ/mol. (a)...
-
Calculate the moment of inertia about the base of composite lamina made up of a semicircle of 120 mm base diameter is removed from base of rectangle 120 mm X 500 mm such that lamina is symmetrical to...
-
Compute cost of goods sold for each of these two companies for the year ended December 31,2009. Computer MerchandisingManufacturing Log Homes 3 Beginning inventory 4 Merchandise 5 Finished goods s...
-
Based on Pereiras determination of recurring and non-recurring expenses for Miland, the companys recurring or core pre-tax earnings last year is c losest to: A . $4.3 billion. B . $4.8 billion. C ....
-
Plaintiff grounds manager sued a manufacturer, Monsanto, alleging that herbicide use caused his non-Hodgkins lymphoma. The jury awarded the plaintiff \($39.3\) million in compensatory damages and...
-
You are provided with the following information for Najera Inc. for the month ended June 30, 2014. Najera uses the periodic method for inventory. Instructions (a) Calculate (i) Ending inventory ,...
-
Path for Ampere's law Plane surface Bulging surface A parallel-plate capacitor with plates of radius R is being charged by the current ic. Recall Ampere's Law B.d-po Although there is no current...
-
John Parsons (123-45-6781) and George Smith (123-45-6782) are 70% and 30% owners, respectively, of Premium, Inc. (11-1111111), a candy company located at 1005 16th Street, Cut and Shoot, TX 77303....
-
Write an equation to represent the combustion of thymol referred to in Exercise 44. Include in this equation the values for U and H. Exercise 44 A 1.397 g sample of thymol, C 10 H 14 O(s) (a...
-
The heat of combustion of propan-2-ol at 298.15 K, determined in a bomb calorimeter, is -33.41 kJ/g. For the combustion of one mole of propan-2-ol, determine (a) U, and (b) r H.
-
Refer to Fig. 20.2. The rod has length L and its position is x at some instant, as shown in the figure. Express your answers in terms of x, L, v, B (the magnetic field magnitude), and R, as needed....
-
Day Inc. has 4,089 shares of 5%, $100 par value cumulative preferred stock and 94,145 shares of $1 par value common stock outstanding at December 31. What is the annual dividend on the preferred...
-
Briefly Explain Accounting nd taxation with examples and CGT and also Stocks? What is the CGT?
-
Mini Ltd leased a machine from Levi Ltd. The lease is for an item of machinery that, at the inception of the lease, has a fair value of $1,298,674. There is a bargain purchase option that Mini Ltd...
-
Mr. X transferred a track of land under sec 85 to Corp X. ACB of the land 100,000 and the FMV of the land is $3,000,000. In return, Mr. X received cash of 2,000,000 and common shares with a PUC and...
-
Assume the market price of a 14-year bond for Margaret Inc. is $1,075, and it has a par value of $1,000. The bond has an annual interest rate of 7% that is paid semiannually. What is the yield to...
-
Using the data in SE2, calculate the cost of ending inventory and cost of goods sold according to the average-cost method under the perpetual inventory system. (Round to the nearest dollar.) In SE2,...
-
In a certain school district, 3% of the faculty use none of their sick days in a school year. Find the probability that 5 faculty members selected at random used no sick days in a given year.
-
Accounting for Accounting Changes and Errors Listed below are various types of accounting changes and errors. ______ 1. Change from FIFO to average cost inventory method. ______ 2. Change due to...
-
Error and Change in Estimate Depreciation Tarkington Co. purchased a machine on January 1, 2007, for $440,000. At that time it was estimated that the machine would have a 10-year life and no salvage...
-
Depreciation Changes On January 1, 2006, McElroy Company purchased a building and equipment that have the following useful lives, salvage value s, and costs. Building, 40-year estimated useful life,...
-
This morning you purchased a stock that just paid an annual dividend of $3.10 per share. You require a return of 9.2 percent and the dividend will increase at an annual growth rate of 4 percent. If...
-
Your company has been approached to bid on a contract to sell 4,950 voice recognition (VR) computer keyboards a year for four years. Due to technological improvements, beyond that time they will be...
-
ANALYSIS OF A PRINT ADVERTISEMENT Find a print advertisement from a magazine (or, alternatively, you can also find an advertisement online) and analyze the advertisement you have chosen by focusing...

Study smarter with the SolutionInn App


