The standard Gibbs energies of formation, f G, for KO 2 (s) and K 2 O(s)
Question:
The standard Gibbs energies of formation, ΔfG°, for KO2(s) and K2O(s) are -240.59 kJ mol‾1 and -322.09 kJ mol‾1, respectively, at 298 K. Calculate the equilibrium constant for the reaction below at 298 K. Is KO2(s) thermodynamically stable with respect to K2O(s) and O2(g) at 298 K?
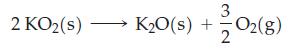
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:





