The standard molar heats of combustion of C(graphite) and CO(g) are -393.5 and -283 kJ/mol, respectively. Use
Question:
The standard molar heats of combustion of C(graphite) and CO(g) are -393.5 and -283 kJ/mol, respectively. Use those data and that for the following reaction
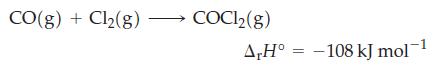
to calculate the standard molar enthalpy of formation of COCl2(g).
Transcribed Image Text:
CO(g) + Cl₂(g) COC1₂(g) A,H° -108 kJ mol-¹ =
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 60% (5 reviews)
Solution To calculate the standard molar enthalpy of formation of COCl2g we can u...View the full answer

Answered By

Atuga Nichasius
I am a Highly skilled Online Tutor has a Bachelor’s Degree in Engineering as well as seven years of experience tutoring students in high school, bachelors and post graduate levels. I have a solid understanding of all learning styles as well as using asynchronous online platforms for tutoring needs. I individualise tutoring for students according to content tutoring needs assessments.
My strengths include good understanding of all teaching methods and learning styles and I am able to convey material to students in an easy to understand manner. I can also assists students with homework questions and test preparation strategies and I am able to help students in math, gre, business , and statistics
I consider myself to have excellent interpersonal and assessment skills with strong teaching presentation verbal and written communication
I love tutoring. I love doing it. I find it intrinsically satisfying to see the light come on in a student's eyes.
My first math lesson that I taught was when I was 5. My neighbor, still in diapers, kept skipping 4 when counting from 1 to 10. I worked with him until he could get all 10 numbers in a row, and match them up with his fingers.
My students drastically improve under my tutelage, generally seeing a two grade level improvement (F to C, C to A, for example), and all of them get a much clearer understanding!
I am committed to helping my students get the top grades no matter the cost. I will take extra hours with you, repeat myself a thousand times if I have to and guide you to the best of my ability until you understand the concept that I'm teaching you.
5.00+
2+ Reviews
10+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
When 1.000 g of gaseous butane, C4H10, is burned at 25oC and 1.00 atm pressure, H2O(l) and CO2(g) are formed with the evolution of 49.50 kJ of heat. a. Calculate the molar enthalpy of formation of...
-
Euler's original article about the Konigsberg Bridge Problem, which is dated 1736, presents a second similar problem with two islands, four rivers flowing around them, and 15 bridges connecting...
-
Zainab company has sold goods on credit RO 55,000 on 31st December 2020 and received RO 15,000 towards credit sales. The company had debit balance of RO 5500 and the balance in accounts receivable...
-
Retailers as well as manufacturers can apply just-in-time (JIT) to their inventory management. Both Best Buy and Circuit City want to know the impact of a JIT inventory system for their operating...
-
In many manufacturing processes, the term work-in-process (often abbreviated WIP) is used. At the LSS Publishing book manufacturing plants, WIP represents the time it takes for sheets from a press to...
-
True or False: For personal investment decision making, rates of return are used more frequently than present worth.
-
The following are selected transactions of Graves Company. Graves prepares financial statements quarterly. Jan. 2 Purchased merchandise on account from Ally Company, $30,000, terms 2/10, n/30....
-
How would you import a relational model design and generate a logical model from a relational model?
-
XYZ is a calendar-year corporation that began business on January 1, 2017. For 2017, it reported the following information in its current year audited income statement. Notes with important tax...
-
Can a chemical compound have a standard enthalpy of formation of zero? If so, how likely is this to occur? Explain.
-
A 1.22 kg piece of iron at 126.5 C is dropped into 981 g water at 22.1 C. The temperature rises to 34.4 C. What will be the final temperature if this same piece of iron at 99.8 C is dropped into 325...
-
Let X = (X 1 , X 2 ) be a two-dimensional normal random vector; E{X 1 } = 1, E{X 2 1 } = 5, E{X 2 } = 2, E{X 2 1 } = 13, E{X 1 X 2 } = 2. (a) Write the density of the centered vector Y = Xm, where...
-
An analyst would like to evaluate the management of company X for their ability to generate profits. The analyst feels that taxes are out of the management's control. Which profitability ratio is...
-
Calculating Percent Change Be sure your answer is a number rounded to one decimal point; do not include percent signs, minus signs, etc. In September 2019, Honda sold 1,541 Insights. In September...
-
As an auditor, you are required to write a letter to a client, you explain the operation of corporation tax by demonstrating an understanding of the underpinning concepts. You must use a range of...
-
In this discussion, we will delve into the fascinating connection between biblical principles and the field of managerial accounting. How would you explain the relationship between biblical...
-
Which 3 of these transactions could be set up as recurring transactions?
-
Dapper Hat Makers Company sells merchandise on credit. During the fiscal year ended July 31, the company had net sales of $2,300,000. At the end of the year, it had Accounts Receivable of $600,000...
-
Find the image of x = k = const under w = 1/z. Use formulas similar to those in Example 1. y| y = 0 -21 -2 -1 -1, /1 12 T -1 -1 y= -2 x =0
-
Morlan Corporation is preparing its December 31, 2010, financial statements. Two events that occurred between December 31, 2010, and March 10, 2011, when the statements were issued, are described...
-
Tina Bailey, a student of intermediate accounting, was heard to remark after a class discussion on segment reporting, All this is very confusing to me. First we are told that there is merit in...
-
Foley Corporation has seven industry segments with total revenues as follows. Penley $600 Cheng $ 225 Konami 650 Takuhi 200 KSC 250 Molina 700 Red Moon 275 Based only on the revenues test, which...
-
discuss the principles of cell-cell communication in multicellular organisms, including direct cell-cell contact through gap junctions and synaptic transmission, as well as paracrine, endocrine, and...
-
What does the prospecting process look like at your organization? Is there a'' calls per day'' metric that the front-line sales professionals are required to make? Why is this important?
-
What are the ecological implications of global climate change on ecosystems, including shifts in species distributions, phenological mismatches, alterations in species interactions, and changes in...

Study smarter with the SolutionInn App


