Two equations can be written for the dissolution of Mg(OH) 2 (s) in acidic solution. (a) Explain
Question:
Two equations can be written for the dissolution of Mg(OH)2(s) in acidic solution.
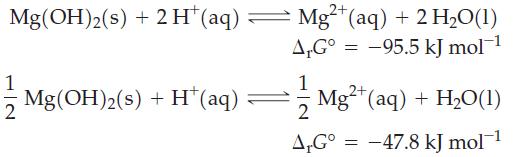
(a) Explain why these two equations have different ΔrG° values.
(b) Will K for these two equations be the same or different? Explain.
Transcribed Image Text:
2+ Mg(OH)2(s) + 2H* (aq) — Mg²+ (aq) + 2 H₂O(1) A.G° -95.5 kJ mol-¹ 1 Mg(OH)2(s) + H* (aq) 1 2 A,Gº = 2+ Mg²+ (aq) + H₂O(1) -47.8 kJ mol-¹ =
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 0% (1 review)
a The two equations have different rG values because they represent different paths for the same chemical reaction The first equation shows the direct ...View the full answer

Answered By

JAPHETH KOGEI
Hi there. I'm here to assist you to score the highest marks on your assignments and homework. My areas of specialisation are:
Auditing, Financial Accounting, Macroeconomics, Monetary-economics, Business-administration, Advanced-accounting, Corporate Finance, Professional-accounting-ethics, Corporate governance, Financial-risk-analysis, Financial-budgeting, Corporate-social-responsibility, Statistics, Business management, logic, Critical thinking,
So, I look forward to helping you solve your academic problem.
I enjoy teaching and tutoring university and high school students. During my free time, I also read books on motivation, leadership, comedy, emotional intelligence, critical thinking, nature, human nature, innovation, persuasion, performance, negotiations, goals, power, time management, wealth, debates, sales, and finance. Additionally, I am a panellist on an FM radio program on Sunday mornings where we discuss current affairs.
I travel three times a year either to the USA, Europe and around Africa.
As a university student in the USA, I enjoyed interacting with people from different cultures and ethnic groups. Together with friends, we travelled widely in the USA and in Europe (UK, France, Denmark, Germany, Turkey, etc).
So, I look forward to tutoring you. I believe that it will be exciting to meet them.
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
In Section 11.3.1, we found the least squares estimators of α and β by a two-stage minimization. This minimization can also be done using partial derivatives. (a) Compute...
-
Water flows between the North American Great Lakes as depicted in Fig. P13.12. Based on mass balances, the following differential equations can be written for the concentrations in each of the lakes...
-
The schematic diagram of the solution process as the net sum of three steps in Figure 13.4 does not show the relative magnitudes of the three components because these will vary from case to case. For...
-
At the current year-end, a company shows the following unadjusted balances for selected accounts. a. After an analysis of future sales discounts, the company estimates that the Allowance for Sales...
-
The controller of Trenshaw Company wants to improve the companys control system by preparing a month-by-month cash budget. The following information is for the month ending July 31, 2014. June 30,...
-
Draw the shear and moment diagrams for the overhangbeam. 4 kN/m B 3m 3m-
-
Quilts R Us (QRU) is considering investing in a new patterning attachment with the cash flow profile shown in the table below. QRU's MARR is 13.5 percent/year. a. What is this investment's external...
-
Completed Contract and Percentage of Completion with Interim Loss Reynolds Custom Builders (RCB) was established in 1985 by Avery Conway and initially built high-quality customized homes under...
-
On June 30, 2016, Flint Limited issued 13.75% bonds with a par value of $802,000 due in 20 years. They were issued at 99 and were callable at 102 at any date after June 30, 2023. Because of lower...
-
Currently, CO 2 is being studied as a source of carbon atoms for synthesizing organic compounds. One possible reaction involves the conversion of CO 2 to methanol, CH 3 OH. With the aid of data from...
-
For the reaction 2 SO 2 (g) + O 2 (g) 2 SO 3 (g), Kc = 2.8 x 10 2 M -1 at 1000 K. (a) What is r G at 1000 K? (b) If 0.40 mol SO 2 0.18 mol O 2 , and 0.72 mol SO 3 are mixed in a 2.50 L flask at...
-
Smith Machining makes three products. The company's annual budget includes $1,000,000 of overhead. In the past, the company allocated overhead based on expected capacity of 40,000 direct labor hours....
-
Least squares is a technique used a. in regression analysis. b. to determine the accuracy of a forecasting model. c. to determine an exponential smoothing model. d. none of the above.
-
Can fuel hedging lower fuel cost? Why do some airlines hedge fuel prices?
-
What is ancillary revenue? What category of airlines realize the highest percentage of ancillary revenue? Why?
-
Air fares between a major hub city and a spoke city are often higher than for another city-pair of comparable distance and city sizes. Why?
-
Trend projection is an example of a. Delphi process. b. time series. c. causal methods. d. regression analysis.
-
Use the accounting equation to compute the missing financial statement amounts (a), (b), and(c). Equity $40,000 70,000 Company Liabilities Assets 75,000 85,000 2 $ (a 25,000 20,000
-
Design an experiment to demonstrate that RNA transcripts are synthesized in the nucleus of eukaryotes and are subsequently transported to the cytoplasm.
-
Weighted-average method, assigning costs (continuation of 17-19). For the data in Exercise 17-19, summarize total costs to account for, calculate cost per equivalent unit for direct materials and...
-
FIFO method, equivalent units. Refer to the information in Exercise 17-19. Suppose the Assembly Division at Fenton Watches, Inc., uses the FIFD method of process costing instead of the...
-
FIFO method, assigning costs (continuation of 11-21). For the data in Exercise 17-19, use the FIFO method to summarize total costs to account for, calculate cost per equivalent unit for direct...
-
Theorists have suggested that there may be a link between childhood trauma and involvement in the criminal justice system. These theorists think that rehabilitation for youth involved in the criminal...
-
I have worked with you before and was very satisfied. Is it possible to get a literature review for a final thesis done on Adverse Effects of "Toxic Nurse Managers' on Nurses. I started it but...
-
1. Judging from your own experience, do you think it was ethical for Dr. Edwards to discuss his former teachers with the new principal? Why, or why not? 2. Curricular changes are often influenced by...

Study smarter with the SolutionInn App


