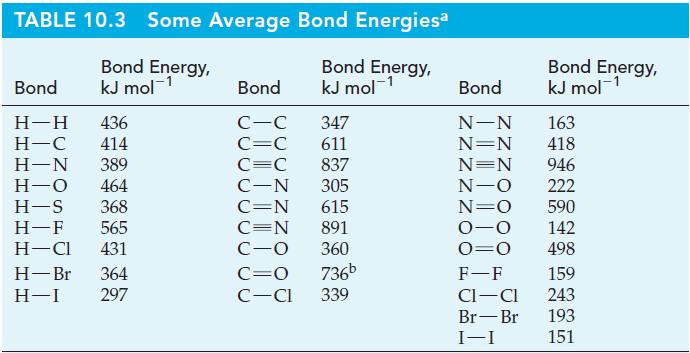
Use bond enthalpies from Table 10.3 to determine whether CH 4 (g), CH 3 OH(g), H 2
Question:
Use bond enthalpies from Table 10.3 to determine whether CH4(g), CH3OH(g), H2CO(g), or HCOOH(g) produces the most energy per gram when burned completely in O2(g) to give CO2(g) and H2O(g). Is there any relationship between the oxidation state of carbon and the heat of combustion (in kJ kg–1 or kJ mol–1)?
Table 10.3
Transcribed Image Text:
TABLE 10.3 Some Average Bond Energiesa Bond Energy, kJ mol-¹ Bond Energy, kJ mol-¹ Bond H-H 436 H-C 414 H-N 389 H-O 464 H-S 368 H-F 565 H-Cl 431 H-Br 364 H-I 297 Bond C-C C=C C=C C-N C=N C=N C-O 347 611 837 305 615 891 360 C=O 736b C-Cl 339 Bond Energy, kJ mol-¹ Bond N-N 163 N=N 418 N=N 946 N-O 222 N=O 590 0-0 142 0=0 498 F-F CI-CI Br-Br I-I 159 243 193 151
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (3 reviews)

Answered By

DAYADAS K G
HAVE A 2 YEAR EXPERIENCE IN THE FIELD OF TEACHING UNDER GRADUATE STUDENTS IN THE STREAM OF BACHELORS OF ENGINEERING IN ELECTRONICS AND COMMUNICATIONS ENGINEERING
Serve and support functional activities of departmental committees
Assist and support senior professors in their day-to-day tasks and functions Assess, review and evaluate student activities and progress
Planning and creating lectures, in-class discussions, and assignments
Attend training sessions or professional meetings to develop or maintain professional knowledge.
SUBJECTS HANDLED: Circuit theory, Basic electrical and electronics , Microprocessors and microcontrollers, Digital signal processing, Optical Communication, Analog and Digital Communication
0.00
0 Reviews
10+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
Methane, CH 4 , can be converted to methanol, which, like ethanol, can be used as a fuel. The energy level diagram shown here presents relationships between energies of the fuels and their oxidation...
-
Use average bond enthalpies from Table 8.4 to estimate the enthalpies of the following gas-phase reactions: Are both reactions exothermic? How do these values relate to the different strengths of...
-
The earth receives 1.8 X 1014 kj/s of solar energy. W'hat mass of solar material is converted to energy over a 24-h period to provide the daily amount of solar energy to the earth? What mass of coal...
-
Count Dracula, the most famous vampire, rumored to have killed at least 200,000 people, was based on a real person who lived in eastern Europe about 600 years ago. He was indeed a "monster," although...
-
Tovar applied for the position of resident physician in Paxton Community Memorial Hospital. The hospital examined his background and licensing and assured him that he was qualified for the position....
-
How can an auditor test to determine whether Receiving Department procedures are applied properly? a. Test a sample of receiving documents. b. Observe receiving procedures on a surprise basis. c....
-
How is a financial audit used for purposes of control?
-
Think about sending a first-class letter to an international pen pal. Describe the process that the letter goes through to get from your initial creation of the letter to being read by your friend,...
-
Calculate the return on investment (as a %) for the given company. (Round your answer to the nearest tenth of a percent.) Company Net Sales Cost of Goods Sold Gross Operating Profit Expenses a...
-
The electron-group geometry of H 2 O is (a) Tetrahedral; (b) Trigonal planar; (c) Bent; (d) Linear; (e) None of these.
-
Which of the following molecules has no polar bonds? (a) H 2 CO; (b) CCl 4 ; (c) OF 2 ; (d) N 2 O; (e) None of these.
-
1. Discuss the problems facing ICRISAT. To what extent are these problems the result of its leadership over the past several years? 2. Describe Dr. Dars leadership experience. What kind of leadership...
-
4. A car leaves point A and drives at 80 km/hr [60Wof N]. The car then heads north at 60 km/hr arriving at point B. If the entire trip lasts 2.5 hours, determine the car's acceleration during the...
-
A 0.950 kg hammer is moving horizontally at 7.00 m/s when it strikes a nail and comes to rest after driving it 1.00 cm into a board. (a) Calculate the duration of the impact in seconds. S + (b) What...
-
Spectators watch a bicycle stunt rider travel off the end of a 57.5 ramp, rise to the top of his trajectory and, at that instant, suddenly push his bike horizontally away from him so that he falls...
-
#2: If 2 masses 7 kg and 17 kg have velocities <6,-10 > and respectively, find the final velocities of the 17 kg objects a) Find the final velocity of the 5 kg object if the collision is elastic...
-
Two bowling balls are at rest on top of a uniform wooden plank with their centers of mass located as in the figure below. The plank has a mass of 4.50 kg and is 1.00 m long. Find the horizontal...
-
Oysters Away shucks and packs oysters and sells them wholesale to fine restaurants across the state. The income statement for last year follows: Pickers, shuckers, and packers are employed on an...
-
Sundial Technologies produces and sells customized network systems in New Brunswick. The company offers a 60-day, all software and labor-and an extra 90-day, parts-only- warranty on all of its...
-
Actual costing, normal costing, accounting for manufacturing overhead. Destin Products uses a job-costing system with two direct-cost categories (direct materials and direct manufacturing labor) and...
-
Job costing, normal and actual costing. Anderson Construction assembles residential houses. It uses a job-costing system with two direct-cost categories (direct materials and direct labor) and one...
-
Budgeted manufacturing overhead rate, allocated manufacturing overhead. Waheed Company uses normal costing. It allocates manufacturing overhead costs using a budgeted rate per machine-hour. The...
-
Contribution Income Statement and Cost-Volume-Profit Graph Kopi Company produces dog cages that are sold for $37 per unit. The company produced and sold 5,600 dog cages during July 2017. There were...
-
Karen and Jeremy, both in their 30s, file a joint tax return for 2016. Karen's wages are $15,000 and Jeremy's wages are $23,000 for the year. Your total adjusted gross income is $38,000; and Jeremy...
-
Part III should be completed before beginning Part IV. Background: Day four: the same student goes into the Coffee House and orders two croissants. The cashier takes the order and asks for a $4...

Study smarter with the SolutionInn App


