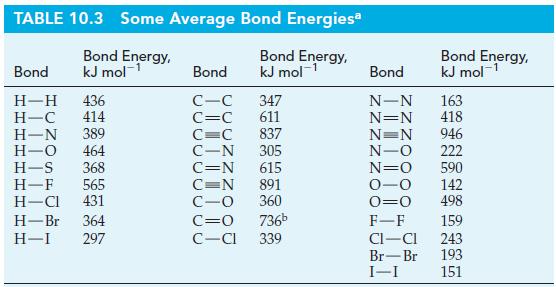
Use the bond-dissociation energies of N 2 (g) and O 2 (g) in Table 10.3, together with
Question:
Use the bond-dissociation energies of N2(g) and O2(g) in Table 10.3, together with data from Appendix D, to estimate the bond-dissociation energy of NO(g).
Table 10.3
Transcribed Image Text:
TABLE 10.3 Some Average Bond Energies* Bond Energy, kJ mol-¹ Bond Energy, kJ mol-¹ Bond H-H 436 H-C 414 H-N 389 H-O 464 H-S 368 H-F 565 H-CI 431 H-Br 364 H-I 297 Bond C-C C=C 347 611 C=C 837 C-N 305 C=N 615 C=N 891 C-O 360 C=O 736b C-Cl 339 Bond N-N N=N N=N N-O N=O 0-0 0=0 F-F CI-CI Br-Br I-I Bond Energy, kJ mol-¹ 163 418 946 222 590 142 498 159 243 193 151
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (3 reviews)
Solution N NO 0 NON0 AH for N N 946KJmol AH ...View the full answer

Answered By

Ashington Waweru
I am a lecturer, research writer and also a qualified financial analyst and accountant. I am qualified and articulate in many disciplines including English, Accounting, Finance, Quantitative spreadsheet analysis, Economics, and Statistics. I am an expert with sixteen years of experience in online industry-related work. I have a master's in business administration and a bachelor’s degree in education, accounting, and economics options.
I am a writer and proofreading expert with sixteen years of experience in online writing, proofreading, and text editing. I have vast knowledge and experience in writing techniques and styles such as APA, ASA, MLA, Chicago, Turabian, IEEE, and many others.
I am also an online blogger and research writer with sixteen years of writing and proofreading articles and reports. I have written many scripts and articles for blogs, and I also specialize in search engine
I have sixteen years of experience in Excel data entry, Excel data analysis, R-studio quantitative analysis, SPSS quantitative analysis, research writing, and proofreading articles and reports. I will deliver the highest quality online and offline Excel, R, SPSS, and other spreadsheet solutions within your operational deadlines. I have also compiled many original Excel quantitative and text spreadsheets which solve client’s problems in my research writing career.
I have extensive enterprise resource planning accounting, financial modeling, financial reporting, and company analysis: customer relationship management, enterprise resource planning, financial accounting projects, and corporate finance.
I am articulate in psychology, engineering, nursing, counseling, project management, accounting, finance, quantitative spreadsheet analysis, statistical and economic analysis, among many other industry fields and academic disciplines. I work to solve problems and provide accurate and credible solutions and research reports in all industries in the global economy.
I have taught and conducted masters and Ph.D. thesis research for specialists in Quantitative finance, Financial Accounting, Actuarial science, Macroeconomics, Microeconomics, Risk Management, Managerial Economics, Engineering Economics, Financial economics, Taxation and many other disciplines including water engineering, psychology, e-commerce, mechanical engineering, leadership and many others.
I have developed many courses on online websites like Teachable and Thinkific. I also developed an accounting reporting automation software project for Utafiti sacco located at ILRI Uthiru Kenya when I was working there in year 2001.
I am a mature, self-motivated worker who delivers high-quality, on-time reports which solve client’s problems accurately.
I have written many academic and professional industry research papers and tutored many clients from college to university undergraduate, master's and Ph.D. students, and corporate professionals. I anticipate your hiring me.
I know I will deliver the highest quality work you will find anywhere to award me your project work. Please note that I am looking for a long-term work relationship with you. I look forward to you delivering the best service to you.
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
Resonance energy is the difference in energy between a real moleculea resonance hybridand its most important contributing structure. To determine the resonance energy for benzene, we can determine an...
-
Basing your answers on the bond dissociation energies in Table 4.3, calculate which of the following reactions are endothermic and which are exothermic: (a) (CH3)2CHOH + HF - (CH3)2CHF + H2O (b)...
-
Use data from Appendix D and other information from this chapter to estimate the temperature at which the dissociation of I 2 (g) becomes appreciable [for example, with the I 2 (g) 50% dissociated...
-
A curve C in three dimensions is given parametrically by (x(t), y(t), z(t)), where t is a real parameter, with a t b. Show that the equation of the tangent line at a point P on this curve where t...
-
Crede Inc. has two divisions. Division A makes and sells student desks. Division B manufactures and sells reading lamps. Each desk has a reading lamp as one of its components. Division A can purchase...
-
Determine the mass moment of inertia of the overhung crank about the x axis. The material is steel having a density of ? = 7.85 Mg/m 3 . 20 mm 30 mm 90 mm 50 mm 180 mm 20 mm 30 mm 30 mm 20 mm 50 mm
-
SWIGART v. BRUNO CALIFORNIA COURT OF APPEALS 13 CAL. APP. 5TH 529 2017 According to the American Endurance Ride Conference, endurance riding is a highly competitive and demanding sport. It is...
-
Jan Volk, financial manager of Green Sea Transport (GST), has been asked by her boss to review GSTs outstanding debt issues for possible bond refunding. Five years ago, GST issued $40,000,000 of 11...
-
Joe is 25 and has a wage of $30,000. He is single with no dependents, and files as single. He rents and makes no contributions to charity. He contributed $800 to a Roth IRA in 2022. What is his Total...
-
Sodium azide, NaN 3 , is the nitrogen gas-forming substance used in automobile air-bag systems. It is an ionic compound containing the azide ion, N 3 - In this ion, the two nitrogen-to-nitrogen bond...
-
Draw Lewis structures for two different molecules with the formula C 3 H 4 . Is either of these molecules linear? Explain.
-
Suppose a playlist you just created has 13 tracks. After listening to the playlist, you decide that you like 5 of the songs. The random feature on your music player will play each of the 13 songs...
-
When and how does a buyer or lessee pay for goods?
-
Under the doctrine of strict liability, defendants are liable for the results of their acts only if they intended those results. (True/False)
-
Under the UCC, acceptance can be made by any means of communication reasonable under the circumstances. (True/False)
-
In a sale on approval, the risk of loss passes to the buyer as soon as the buyer takes possession. (True/False)
-
Russo contracts with Playlist, Inc., to create a website through which users can post and share movies, music, and other forms of digital entertainment. Russo goes to work. Before the site is online,...
-
Gabrielle Kramer, owner of Pet Paradise, is opening a new store in Columbus, Ohio. Her major concern is the hiring of a manager and several associates who are animal lovers. She also has to...
-
Heineken N.V., a global brewer based in the Netherlands, reports the following balance sheet accounts for the year ended December 31, 2016 (euros in millions). Prepare the balance sheet for this...
-
Strategy, balanced scorecard, Service Company Snyder Corporation is a small information systems consulting firm that specializes in helping companies implement sales-management software. The market...
-
Strategic analysis of operating income (continuation of 13-26). Refer to Exercise 13-26. 1. Calculate the operating income of Snyder Corporation in 2008 and 2009. 2. Calculate the growth,...
-
Analysis of growth, price-recovery, and productivity components (continuation of 13-27). Suppose that during 2009 the market for implementing sales-management software increases by 5% and that Snyder...
-
Suppose a company has 50 million shares outstanding at a price of $52.14 per share. The market value of its debt is $17 billion. What is the market value of its assets in billions of dollars
-
Deemer Corporation has an activity-based costing system with three activity cost pools--Processing, Supervising, and Other. In the first stage allocations, costs in the two overhead accounts,...
-
The assembly department of Oriole Furniture Company has the following production and manufacturing cost data f January 2022, the first month of operation: 1. Production: 18,200 units finished and...

Study smarter with the SolutionInn App


