Use the following electrode potential diagram for basic solutions to classify each of the statements below as
Question:
Use the following electrode potential diagram for basic solutions to classify each of the statements below as true or false. Assume standard conditions.
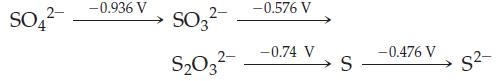
(a) Sulfate (SO42–) is a stronger oxidant than thiosulfate (S2O32–) in basic solution.
(b) S2– can be used as a reducing agent in basic solutions.
(c) S2O32– is stable with respect to disproportionation to SO32– and S in basic solution.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:





