Use the information given here, data from Appendix D, and equation (7.22) to calculate the standard enthalpy
Question:
Use the information given here, data from Appendix D, and equation (7.22) to calculate the standard enthalpy of formation per mole of ZnS(s).
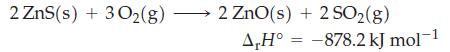
Eq.7.22
![A.H = [cx AHC + dx AHD +...] [ax AHA + bx AHB + .] (7.22) weighted sum of AcH values for the products](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1699/6/0/4/689654de8d1674691699604684867.jpg)
Transcribed Image Text:
2 ZnS(s) + 3O₂(g) 2 ZnO(s) + 2 SO₂(g) A,H° -878.2 kJ mol-1
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (3 reviews)
2ZnSs 302g 2ZnO2SO2g AH rxn...View the full answer

Answered By

OTIENO OBADO
I have a vast experience in teaching, mentoring and tutoring. I handle student concerns diligently and my academic background is undeniably aesthetic
4.30+
3+ Reviews
10+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
Calculate the standard enthalpy of formation of NOCI (g) from the enthalpy of formation of NO given in Table 2.5, together with the following information: 2 NOCl (g) 2 NO (g) + Clz (g) 1 Ho = + Uo=...
-
Calculate the standard enthalpy of formation for diamond, given that C(graphite) + O2(g) CO2(g) Afr =-393.5 kJ/mol C(diamond) + O2(g) CO2(g) AF1 395.4 kJ/mol
-
The average energy output of a good grade of coal is 2.6 10 7 kJ/ton. Fission of 1 mol of 235 U releases 2.1 10 10 kJ. Find the number of tons of coal needed to produce the same energy as 1 lb of...
-
You have obtained the following share prices from Yahoo Finance. Company 1 is listed on the London Stock Exchange. You want to estimate the beta value for Company 1. You run a regression of the share...
-
The following calendar year-end information is taken from the December 31, 2009, adjusted trial balance and other records of Gucci Company. Advertising expense . . . . . . . . . . . . . . . . . . . ....
-
Two steel wires and a copper wire support a load of \(50 \mathrm{kN}\) as shown in Fig. 13.51. The diameter of steel wire is \(20 \mathrm{~mm}\) and that of copper wire \(15 \mathrm{~mm}\). If...
-
How do you write a Problem Statement?
-
John Clais began operations as an event consultant on January 1, 2008. The trial balance columns of the work sheet on March 31 are as follows. Other data: 1. Supplies on hand total $500. 2....
-
What is strategic management? What is strategic planning? What is strategic thinking? What is the difference between strategic planning, strategic management and strategic thinking? How are strategic...
-
Nucor Corporation produces steel and steel products at its eight mills and is a major recycler of scrap metal. The following data relate to Nucor for four years. In 2017, Nucors net income was higher...
-
Use the data in Figure 7-18 and information to establish possible relationships between the molecular structure of the hydrocarbons and their standard enthalpies of formation. Figure 7-18 Positive...
-
The standard enthalpy of fermentation of glucose to ethanol is Use the standard enthalpy of combustion for glucose to calculate the enthalpy of combustion for ethanol. C6H12O6(s)- 2 CH3CHOH(1) + 2...
-
How do you incorporate the contents of a JavaScript source file into an HTML document?
-
Change the total fixed manufacturing overhead cost for the Milling Department in Data area back to $390,000, keeping all of the other data the same as in the original example. Consider a new job, Job...
-
BuyCo, Inc., holds 29 percent of the outstanding shares of Marqueen Company and appropriately applies the equity method of accounting. Excess cost amortization (related to a patent) associated with...
-
Tom selis mutual funds on a graduated commission structure. He receives 3.1% on the first $40,000 of sales in a month 4.2% on the next $40,000, and 5.3% on all further sales. What are his gross...
-
A hamburger factory produces 55,000 hamburgers each week. The equipment used costs $15,000 and will remain productive for three years. The labor cost per year is $14,500. a. What is the productivity...
-
a. Purchased $78,000 of raw materials on account. b. $76,000 in raw materials were used in production. Of this amount, $62,000 was direct materials and the remainder was indirect materials. c. Paid...
-
Southwest Airlines is able to keep fares low, in part because of relatively low maintenance costs on its airplanes. One of the main reasons for the low maintenance costs is that Southwest flies only...
-
Modify the counter from Exercise 5.44 such that the counter will either increment by 4 or load a new 32-bit value, D, on each clock edge, depending on a control signal Load. When Load = 1, the...
-
State how each of the following items is reflected in the financial statements. (a) Change from FIFO to LIFO method for inventory valuation purposes. (b) Charge for failure to record depreciation in...
-
Discuss briefly the three approaches that have been suggested for reporting changes in accounting principles.
-
Identify and describe the approach the FASB requires for reporting changes in accounting principles.
-
ACE CONSTRUCTION COMPANY Unadjusted Trial Balance June 30 Number Account Title 101 Cash Debit Credit $ 19,000 126 Supplies 7,000 128 Prepaid insurance 167 168 Equipment Accumulated...
-
The Blue Seas Company, which is under contract to the U.S. Navy, assembles troop deployment boats. As part of its research program, it completes the assembly of the fir of a new model (PT109) of...
-
Matador Company reports net income of $150,000 each year and declares an annual cash dividend of $50,000. The company holds net assets of $1,800,000 on January 1, 2017. On that date, Zelzah purchases...

Study smarter with the SolutionInn App


