Which of the following has the highest molar solubility? (a) MgF2, Ksp = 3.7 10-8; (b)
Question:
Which of the following has the highest molar solubility?
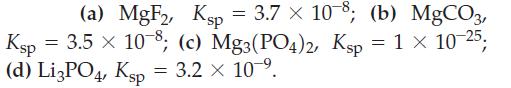
Transcribed Image Text:
(a) MgF2, Ksp = 3.7 × 10-8; (b) MgCO3, Ksp = 3.5 × 10-8; (c) Mg3(PO4)2, Ksp = 1 × 10-25; (d) Li3PO4, Ksp = 3.2 × 10-⁹.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (2 reviews)
To determine which of the given compounds has the highest molar solubility we can compare their solu...View the full answer

Answered By

Kennedy Odhiambo
As a professional writer, I have been in the field for over 5 years having worked as a lecture in different tertiary institutions across the world. With this impeccable experience, I assure provision of a good and supporting environment for students to learn.
5.00+
2+ Reviews
10+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
The following questions focus on the exchange rate between the Russian ruble and the Mexican peso. Assume the exchange rate is flexible. The exchange rate is defined as the number of rubles you must...
-
The mortgage rate charged by a random sample of 36 banks in Clay Town has a sample mean=10.45 and sample standard deviation=0.48. At =0.05, test the claim that the mean mortgage rate equals 11....
-
If x = molar solubility, K sp = solubility product, which of the following compounds would have the relationship: 27x4 = K sp ? a) Bi2S3 b) Ag2CO3 c) AgCl d) PbI2 e) Ag3PO4
-
A molecule with the molecular formula C11H12N2O2 (relative molecular mass = 204.23) crystallized to form monoclinic crystals with a=18.899 , b=5.7445 , and c=9.309 , with =101.776. The crystal...
-
Refer to Practice 20-5. Compute TOTAL income taxes payable, after the change to FIFO is made, as of December 31, 2009, December 31, 2010, and December 31, 2011. Recall that the change to FIFO will...
-
GATT and its successor, the World Trade Organization, have established a set of rules for the commercial conduct of trading nations. Explain.
-
Refer to the information in Exercise 24-3 and assume instead that double-declining depreciation is applied. Compute the machines payback period (ignore taxes). (Round the payback period to three...
-
Jamie Peters invested $100,000 to set up the following portfolio one year ago: a. Calculate the portfolio beta on the basis of the original cost figures. b. Calculate the percentage return of each...
-
When President Obama was President he had discussed raising income taxes for individuals earning over $250,000 in income. Explain how these higher income taxes will affect the aggregate demand curve....
-
Saturated solutions of sodium phosphate, copper(II) chloride, and ammonium acetate are mixed together. The precipitate is (a) Copper(II) acetate; (b) Copper(II) phosphate; (c) Sodium chloride; (d)...
-
Lead(II) chloride is most soluble in (a) 0.100 M NaCl; (b) 0.100 Na 2 S 2 O 3 ; (c) 0.100 M Pb(NO 3 ) 2 ; (d) 0.100 M NaNO 3 ; (e) 0.100 MnSO 4 .
-
A 2.05-m-tall basketball player takes a shot when he is 6.02 m from the basket (at the three-point line). If the launch angle is 25o and the ball was launched at the level of the players head, what...
-
The property tax on a farm worth $880,000 was $8,250. What percentage of tax was paid?
-
Quincy, age 79, had a balance in his IRA account on December 31 of the prior year of $400,000. What is his required minimum distribution (RMD)?
-
What kind of actions could they take in order to reduce working capital requirements?
-
LIFO is a somewhat controversial inventory costing method that is used in the U.S. but is not allowed by International Financial Reporting Standards (IFRS). It is one of the main barriers to full...
-
How does a modern operating system efficiently manage memory resources through techniques such as virtual memory, paging, and segmentation?
-
For each of the following independent situations, indicate the apparent internal control weaknesses and suggest alternative procedures to eliminate the weaknesses. 1. John Smith is the petty cash...
-
Recall that Chapter 8 described the binary search algorithm for finding a particular entry in an ordered list. The idea behind binary search is to begin looking in the exact center of the list. If...
-
Accounting is ingrained in our society and it is vital to our economic system. Do you agree? Explain.
-
Identify and describe the steps in the accounting process.
-
(a) Who are internal users of accounting data? (b) How does accounting provide relevant data to these users?
-
The Workers Compensation and Injury Management Act 1981 (WA), provides compensation for workers injured (along with certain dependents in the event of a workers death) on a no fault basis for...
-
What are the environment policy of your company, how your company develop alternative strategies in order to act environmental friendly?
-
ation costs increase the cost of new equity. True false question. True False

Study smarter with the SolutionInn App


