Without performing detailed calculations, indicate whether equilibrium is displaced either far to the left or far to
Question:
Without performing detailed calculations, indicate whether equilibrium is displaced either far to the left or far to the right for each of the following reactions. Use data from Appendix D as necessary.
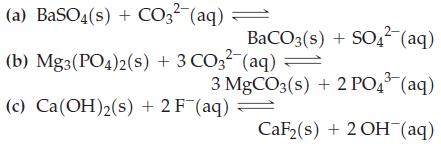
Transcribed Image Text:
(a) BaSO4(s) + CO3²-(aq) = (b) Mg3(PO4)2(s) + 3 CO3² (aq) = BaCO3(s) + SO4²- (aq) 3 MgCO3(s) + 2 PO43³ (aq) CaF₂(s) + 2OH(aq) (c) Ca(OH)2(s) + 2F (aq) =
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (3 reviews)

Answered By

Mugdha Sisodiya
My self Mugdha Sisodiya from Chhattisgarh India. I have completed my Bachelors degree in 2015 and My Master in Commerce degree in 2016. I am having expertise in Management, Cost and Finance Accounts. Further I have completed my Chartered Accountant and working as a Professional.
Since 2012 I am providing home tutions.
3.30+
2+ Reviews
10+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
Without performing detailed calculations, indicate why you would expect each of the following reactions to occur to a significant extent as written. Use data from Appendix D as necessary. (a)...
-
Planning is one of the most important management functions in any business. A front office managers first step in planning should involve determine the departments goals. Planning also includes...
-
Managing Scope Changes Case Study Scope changes on a project can occur regardless of how well the project is planned or executed. Scope changes can be the result of something that was omitted during...
-
Accurate Job Costing must be done on three levels. Which of the following is not one of these levels? Tracking and controlling costs during jobs Tracking gross profit each month Filing records on...
-
Refer to PE 46. On December 31, Greg received a statement from the newspaper publisher notifying him that he had earned $13,700 for his December deliveries. Because December 31 is the end of Gregs...
-
How is the noncontrolling interest in a subsidiary company calculated as of the end of the current year?
-
The company is committed to ethical conduct and has no tolerance for fraud and unethical behavior. There are some concerns about abuses in your department. Do you know anything about the concerns I...
-
Special Order Louisville Corporation produces baseball bats for kids that it sells for $32 each. At capacity, the company can produce 50,000 bats a year. The costs of producing and selling 50,000...
-
The price of a laser printer purchased by Paul's Printers and Office Supplies was $713. It cost $27 for delivery. The salvage value at the end of a 5-year life is $65. what is the depreciation...
-
In the manner used to construct Figure 21-5, complete the diagram outlined. Specifically, indicate the reactants (and conditions) you would use to produce the indicated substances from Ca(OH) 2 ....
-
The empirical formula of the mineral beryl is Be 3 Al 2 Si 6 O 18 . By using the several descriptions of silicate minerals just given as a guide, describe the structure of the silicate anion in beryl.
-
If Wild Widgets, Inc., were an all-equity company, it would have a beta of .95. The company has a target debt-equity ratio of .40. The expected return on the market portfolio is 11 percent, and...
-
The New Zealand dollar to U.S. dollar exchange rate is 1.38, and the British pound to U.S. dollar exchange rate is 0.65. If you find that the British pound to New Zealand dollar is trading at 0.5,...
-
Calculate the overvaluation of the Thai baht (THB) if you can get 34.6 THB per USD at the exchange counter, but a lunch menu that costs 25 USD in Boston sells for 948.25 THB in Bangkok.
-
If the Canadian dollar to U.S. dollar exchange rate is 1.24 and the British pound to U.S. dollar exchange rate is 0.68, what must be the Canadian dollar to British pound exchange rate?
-
A German sports car is selling for 65,000. What is the dollar price in the United States for the German car if the exchange rate is 0.80 euro per dollar?
-
Why is international regulatory cooperation important? What forms has it taken in the aftermath of the 20072009 financial crisis?
-
During the current year, Benjamin and Valerie were notified that their 2009 tax return was being audited. The IRS commissioner has disallowed all the losses attributable to Valeries cattle breeding...
-
Sandcastles, Inc.s management has recently been looking at a proposal to purchase a new brick molding machine. With the new machine, the company would not have to buy bricks. The estimated useful...
-
In recent years, Walz Company has purchased three machines. Because of frequent employee turnover in the accounting department, a different accountant was in charge of selecting the depreciation...
-
Rogers Corporation purchased machinery on January 1, 2012, at a cost of $250,000. The estimated useful life of the machinery is 4 years, with an estimated salvage value at the end of that period of...
-
Franz Company was organized on January 1. During the first year of operations, the following plant asset expenditures and receipts were recorded in random order. Debits 1. Cost of real estate...
-
5. Mabel's Labels' website was very focused on moms, its target market. Now that products are available in retail stores, should it consider targeting other segments that might have different uses...
-
How do reception and reader-response theories investigate the role of the reader in the interpretation and meaning-making of literary texts, considering factors such as reception history, cultural...
-
what must happen before the office of management (OMB) can apportion funds to your agency? A. The finance office must obligate funds for specific acquisitions B. Your agency must allocate funds to...

Study smarter with the SolutionInn App


