Without performing detailed calculations, show that significant disproportionation of AuCl occurs if you attempt to make a
Question:
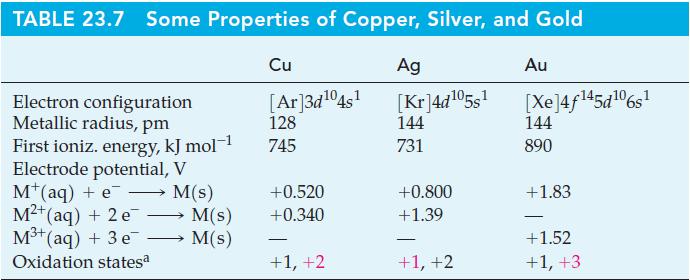
Without performing detailed calculations, show that significant disproportionation of AuCl occurs if you attempt to make a saturated aqueous solution. Use data from Table 23.7 and Ksp(AuCl) = 2.0 x 10–13.
Table 23.7
Transcribed Image Text:
TABLE 23.7 Some Properties of Copper, Silver, and Gold Cu Electron configuration Metallic radius, pm First ioniz. energy, kJ mol-1 Electrode potential, V M+ (aq) + e M²+(aq) + 2 e M³+ (aq) + 3 e Oxidation statesa M(s) M(s) M(s) [Ar]3d¹04s¹ 128 745 +0.520 +0.340 +1, +2 Ag [Kr]4d¹05s¹ 144 731 +0.800 +1.39 +1, +2 Au [Xe]4f145d106s1 144 890 +1.83 - +1.52 +1, +3
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (2 reviews)

Answered By

Ashington Waweru
I am a lecturer, research writer and also a qualified financial analyst and accountant. I am qualified and articulate in many disciplines including English, Accounting, Finance, Quantitative spreadsheet analysis, Economics, and Statistics. I am an expert with sixteen years of experience in online industry-related work. I have a master's in business administration and a bachelor’s degree in education, accounting, and economics options.
I am a writer and proofreading expert with sixteen years of experience in online writing, proofreading, and text editing. I have vast knowledge and experience in writing techniques and styles such as APA, ASA, MLA, Chicago, Turabian, IEEE, and many others.
I am also an online blogger and research writer with sixteen years of writing and proofreading articles and reports. I have written many scripts and articles for blogs, and I also specialize in search engine
I have sixteen years of experience in Excel data entry, Excel data analysis, R-studio quantitative analysis, SPSS quantitative analysis, research writing, and proofreading articles and reports. I will deliver the highest quality online and offline Excel, R, SPSS, and other spreadsheet solutions within your operational deadlines. I have also compiled many original Excel quantitative and text spreadsheets which solve client’s problems in my research writing career.
I have extensive enterprise resource planning accounting, financial modeling, financial reporting, and company analysis: customer relationship management, enterprise resource planning, financial accounting projects, and corporate finance.
I am articulate in psychology, engineering, nursing, counseling, project management, accounting, finance, quantitative spreadsheet analysis, statistical and economic analysis, among many other industry fields and academic disciplines. I work to solve problems and provide accurate and credible solutions and research reports in all industries in the global economy.
I have taught and conducted masters and Ph.D. thesis research for specialists in Quantitative finance, Financial Accounting, Actuarial science, Macroeconomics, Microeconomics, Risk Management, Managerial Economics, Engineering Economics, Financial economics, Taxation and many other disciplines including water engineering, psychology, e-commerce, mechanical engineering, leadership and many others.
I have developed many courses on online websites like Teachable and Thinkific. I also developed an accounting reporting automation software project for Utafiti sacco located at ILRI Uthiru Kenya when I was working there in year 2001.
I am a mature, self-motivated worker who delivers high-quality, on-time reports which solve client’s problems accurately.
I have written many academic and professional industry research papers and tutored many clients from college to university undergraduate, master's and Ph.D. students, and corporate professionals. I anticipate your hiring me.
I know I will deliver the highest quality work you will find anywhere to award me your project work. Please note that I am looking for a long-term work relationship with you. I look forward to you delivering the best service to you.
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
Detailed calculations show that the value of Zeff for the outermost electrons in Na and K atoms is 2.51+ and 3.49+, respectively. (a) What value do you estimate for Zeff experienced by the outermost...
-
Detailed calculations show that the value of Zeff for the outermost electrons in Si and Cl atoms is 4.29+ and 6.12+, respectively. (a) What value do you estimate for Zeff experienced by the outermost...
-
Use the systematic treatment of equilibrium to find the concentrations of the major species in a saturated aqueous solution of LiF. Consider these reactions: (a) Initially, set the ionic strength to...
-
1. Does Amazon have a special responsibility to their employees given the current COVID-19 pandemic? Are they meeting this responsibility? Why or why not? 2. How should Amazon prioritize their...
-
As the accountant for Synergy Solutions, you are in the process of preparing the income statement for the year ended December 31, 2012. In doing so, you have noticed that merchandise costing $10,500...
-
Assume that scenario (b) in Problem 7- 36 is a material weakness. Prepare a draft of the auditors report for an audit of ICFR. Assume that First Coasts auditor is issuing a combined report for the...
-
Bernardino Santos-Rodiguez was piloting a boat owned by his friend, Raul Viera-Torres, in waters near Guayama, Puerto Rico. The boat was equipped with a hydraulic steering system manufactured by...
-
The Vang Hotel opened for business on May 1, 2012. Here is its trial balance before adjustment on May 31. Other data:1. Insurance expires at the rate of $450 per month.2. A count of supplies shows...
-
Prove that for all x = R, if a, b = Z such that x = a + b2, then a and b are unique.
-
Equation (23.18), which represents the chromatedichromate equilibrium, is actually the sum of two equilibrium expressions. The first is an acidbase reaction, H + + CrO 4 2 HCrO 4 . The second...
-
Attempts to make CuI 2 by the reaction of Cu 2+ (aq) and I (aq) produce CuI(s) and I 3 (aq) instead. Without performing detailed calculations, show why this reaction should occur. 2 Cu+ (aq) + 51...
-
Visit www.aarp.com and determine the issues of the senior citizen segment. Are there any to which marketers need to pay attention? Determine how marketers can address them?
-
Suppose that you work at the statistical office of a given country. The graph plots estimates of the labor and capital income shares for that country over time. Your boss suggests that a Cobb-Douglas...
-
What does each column of a RACI chart depict?
-
Which industry is more highly concentrated: one with a Herfindahl index of 1,200 or one with a four-firm concentration ratio of 55 percent?
-
Draw a use case diagram for the situation described in Problem and Exercise 9. Problem and Exercise 9 Starting with a context diagram, draw as many nested DFDs as you consider necessary to represent...
-
An individual files an income tax return for the calendar-year 2018 on September 20, 2019, and pays $1,200, which is the balance of the tax due. Disregarding interest, how much in delinquency...
-
Michael owns a hair salon. During the current year, a tornado severely damages the salon and destroys his personal automobile, which is parked outside. It costs Michael $12,000 to make the necessary...
-
Write an essay describing the differing approaches of nursing leaders and managers to issues in practice. To complete this assignment, do the following: 1. Select an issue from the following list:...
-
Here is the ledger for Stampfer Co. Instructions(a) Reproduce the journal entries for only the transactions that occurred on October 1,10, and 20, and provide explanations for each.(b) Prepare a...
-
The bookkeeper for Bullwinkle Corporation made these errors in journalizing and posting.1. A credit posting of $400 to Accounts Receivable was omitted.2. A debit posting of $750 for Prepaid Insurance...
-
The accounts in the ledger of Roshek Delivery Service contain the following balances on July 31, 2012. Instructions(a) Prepare a trial balance with the accounts arranged as illustrated in the...
-
New Endor Airlines Database New Endor Airlines (NEA) is the premier aviation service in the small island nation of New Endor, located in the southwestern Pacific Ocean. For the 40 years since its...
-
Simpson Staffing Company, founded 10 years ago by Suzy Simpson, recently completed a study of its employee hiring and retention over the last two years. Suzy is keen on using the results of this...
-
What, in your opinion, are the three main problems, which could be studied through management sciences and entrepreneurship, and which the company faces, has faced or will face?

Study smarter with the SolutionInn App


