Chemical reactions are run in each of the beakers depicted below (labeled A, B, and C). The
Question:
Chemical reactions are run in each of the beakers depicted below (labeled A, B, and C). The magnitude and direction of heat and work for each reaction are represented as arrows, with the length of an arrow depicting the relative magnitude of the heat or work.
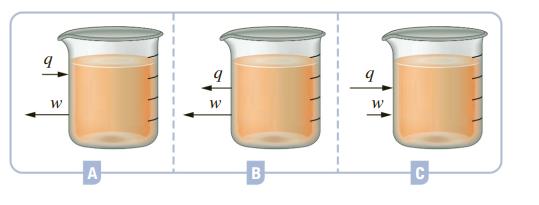
For the reaction in each beaker, answer the following and explain your reasoning:
a. Is the reaction endothermic or exothermic?
b. What is the sign (+ or –) of the work?
c. What is the sign (+ or –) of the enthalpy of each reaction?
d. Is there an increase or decrease in internal energy?
e. What is the temperature of the reaction mixture immediately after the reaction when compared to room temperature?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





