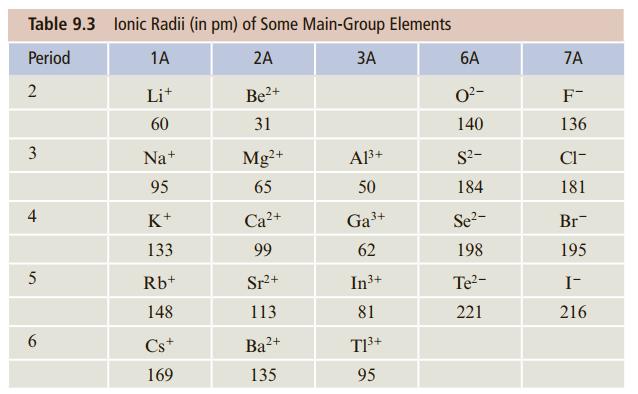
Without looking at Table 9.3, arrange the following ions in order of increasing ionic radius: Sr 2+
Question:
Without looking at Table 9.3, arrange the following ions in order of increasing ionic radius: Sr2+, Mg2+, Ca2+. (You may use a periodic table.)
Transcribed Image Text:
Table 9.3 lonic Radii (in pm) of Some Main-Group Elements Period 1A 2A ЗА 6A 7A Li+ Be2+ O2- F- 60 31 140 136 Na+ Mg2+ A3+ S?- Cl- 95 65 50 184 181 4 K+ Ca2+ Ga+ Se?- Br 133 99 62 198 195 5 Rb+ Sr2+ In3+ Te- I- 148 113 81 221 216 6. Cs+ Ba2+ TI3+ 169 135 95
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 72% (11 reviews)
The ionic radii increase down a...View the full answer

Answered By

Utsab mitra
I have the expertise to deliver these subjects to college and higher-level students. The services would involve only solving assignments, homework help, and others.
I have experience in delivering these subjects for the last 6 years on a freelancing basis in different companies around the globe. I am CMA certified and CGMA UK. I have professional experience of 18 years in the industry involved in the manufacturing company and IT implementation experience of over 12 years.
I have delivered this help to students effortlessly, which is essential to give the students a good grade in their studies.
3.50+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Arrange the following bonds in order of increasing stretching frequencies, and explain your reasoning. C=C C=C C=0 C-C
-
Arrange the following compounds in order of increasing boiling point. (b) (a) (d) (c)
-
Arrange the following compounds in order of increasing melting point. CaO HC-C-O-C-C-H i441 H-C-C-C-C-O- KCI
-
In Exercises 6780, begin by graphing the square root function, f(x) = x. Then use transformations of this graph to graph the given function. h(x) = x + 1 1 Vx+1-1
-
A corporate cash manager who often invests her firm's excess cash in the Eurodollar market is considering the possibility of investing $20 million for 180 days directly in a Eurodollar CD at 6.15...
-
Cintas Corporation is the largest uniform supplier in North America. Selected information from its annual report follows. For the 2016 fiscal year, the company reported sales revenue of $4.9 billion...
-
The financial statements for the business of Autocare are shown below. Required (a) Prepare the statement of cash flows for Autocare for the year ended 30 June 2025, using the direct method. (b)...
-
Suppose that business travelers and vacationers have the following demand for airline tickets from New York to Boston: a. As the price of tickets rises from $200 to $250, what is the price elasticity...
-
Actus reus usually depends on proof of a voluntary act or an omission. Mens rea is also required for criminal liability. Scenario 1: Alphonso believed that Francine, a work colleague had stolen his...
-
A store maintains data on customers, products and purchase records in three tables: CUSTOMER, PRODUCT, PURCHASE. The store manager wants to know which product is on its maximum discount for each...
-
Using electronegativities, decide which of the following bonds is most polar: CO, CS, HBr.
-
Why does sodium chloride normally exist as a crystal rather than as a molecule composed of one cation and one anion?
-
In Exercises determine the work done by the constant force. The locomotive of a freight train pulls its cars with a constant force of 9 tons a distance of one-half mile.
-
In early January, Burger Mania acquired 100% of the common stock of the Crispy Taco restaurant chain. The purchase price allocation included the following items: $4 million, patent; $3 million,...
-
A sled plus passenger with total mass 39 kg is pulled 17 m across the snow (k = 0.19) at a constant velocity by a force directed 25 above the horizontal. a. Calculate the work of the applied force W...
-
The total sales S (in thousands of games) of a video game t months after the game is introduced are given by S(t) = 150t (A) Find S'(t). 1+3 (B) Find S(12) and S'(12). Write a brief interpretation of...
-
1. A ball with mass m is thrown at speed v from zero height on level ground. (a) At what angle should it be thrown so that the area under the trajectory is maximum? (b) At what angle should it be...
-
Find 2x (A) f'(x) for f(x)=- x+3 13-31 (B) y' for y t-4 d 2+3 (C) in two ways dx x
-
If traditional healthcare was to stray into the realm of socioeconomics, what effects on health economic models would you anticipate?
-
Diamond Walker sells homemade knit scarves for $25 each at local craft shows. Her contribution margin ratio is 60%. Currently, the craft show entrance fees cost Diamond $1,500 per year. The craft...
-
Give the type of colloid (aerosol, foam, emulsion, sol, or gel) that each of the following represents. a. Rain cloud b. Milk of magnesia c. Soapsuds d. Silt in water
-
Give the type of colloid (aerosol, foam, emulsion, sol, or gel) that each of the following represents. a. Ocean spray b. Beaten egg white c. Dust cloud d. Salad dressing
-
A gaseous mixture consists of 80.0 mole percent N2 and 20.0 mole percent O2 (the approximate composition of air). Suppose water is saturated with the gas mixture at 25C and 1.00 atm total pressure,...
-
1. Write a Java program that will prompt the user for a number and print out a square with those dimensions. For example, if the user enters 5, return the following: * * * ** ** * * * * * * * * * * *...
-
2. Vector multiplication or dot product is performed by multiplying corresponding elements and summing the products. It can be represented mathematically as oa; *bi where a and b are vectors of...
-
The following data was collected from an experiment testing the hypothesis: The density of cream whipped for 5 minutes increases at higher altitudes. What would be an acceptable interpretation of...

Study smarter with the SolutionInn App


