You perform the hypothetical reaction of an element, X 2 (g), with another element, Y(g), to produce
Question:
You perform the hypothetical reaction of an element, X2(g), with another element, Y(g), to produce XY(g).
a. Write the balanced chemical equation for the reaction.
b. If X2 and Y were mixed in the quantities shown in the container on the left below and allowed to react, which of the three options is the correct representation of the contents of the container after the reaction has occurred?
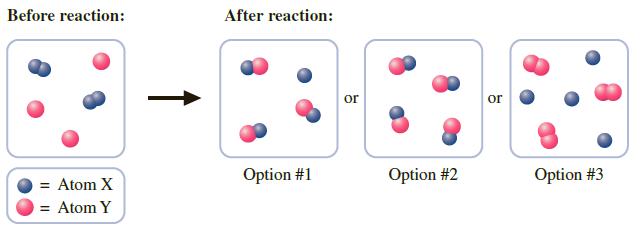
c. Using the information presented in Part b, identify the limiting reactant.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





