(a) Estimate the diffusivity of ethanol in water at (298 mathrm{~K}) when the mol fraction of ethanol...
Question:
(a) Estimate the diffusivity of ethanol in water at \(298 \mathrm{~K}\) when the mol fraction of ethanol in solution is 40\%. Under these conditions (Hammond and Stokes, 1953),
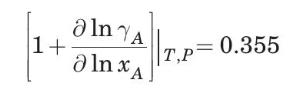
The experimental value reported by Hammond and Stokes (1953) is \(0.42 \times 10^{-5}\) \(\mathrm{cm}^{2} / \mathrm{s}\). The critical volume of ethanol is \(167.1 \mathrm{~cm}^{3} / \mathrm{mol}\); the viscosity of water at \(298 \mathrm{~K}\) is \(0.91 \mathrm{cP}\).
(b) Estimate the diffusivity of acetone in water at \(298 \mathrm{~K}\) when the mol fraction of acetone in solution is \(65 \%\). For this system at \(298 \mathrm{~K}\), the activity coefficient for acetone is given by the Wilson equation (Smith et al., 1996):
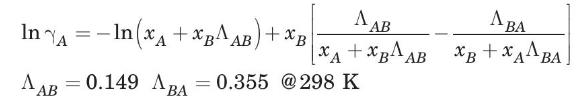
The critical volume for acetone is \(209 \mathrm{~cm}^{3} / \mathrm{mol}\).
Step by Step Answer:






