A solution of carbon tetrachloride and carbon disulfide containing (50 mathrm{wt} %) each is to be continuously
Question:
A solution of carbon tetrachloride and carbon disulfide containing \(50 \mathrm{wt} \%\) each is to be continuously fractionated at standard atmospheric pressure at the rate of \(5000 \mathrm{~kg} / \mathrm{h}\). The distillate product is to contain \(95 \mathrm{wt} \%\) carbon disulfide, the residue \(2.5 \mathrm{wt} \%\). The feed will be \(30 \mathrm{~mol} \%\) vaporized before it enters the tower. A total condenser will be used, and reflux will be returned at the bubble point. VLE data at \(1 \mathrm{~atm}\) (Treybal, 1980), \(x, y^{*}=\mathrm{mol}\) fraction of \(\mathrm{CS}_{2}\), are as follows:
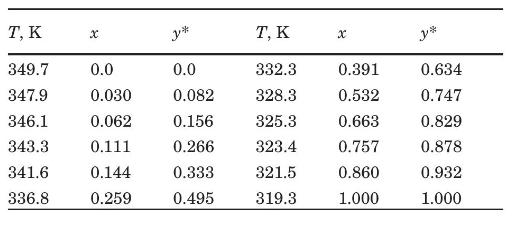
(a) Determine the product rates, in \(\mathrm{kg} / \mathrm{h}\).
(b) Determine the minimum number of theoretical stages required.
(c) Determine the minimum reflux ratio.
(d) Determine the number of theoretical trays required, and the location of the feed tray, at a reflux ratio equal to 1.5 the minimum.
(e) Estimate the overall tray efficiency of a sieve-tray tower of "conventional design" and the number of real trays. The viscosity of liquid \(\mathrm{CCl}_{4}\) and liquid \(\mathrm{CS}_{2}\) as functions of temperature are given by equation (6-42) with:
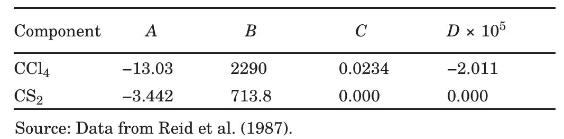
Data From Equation 6-42:-

Step by Step Answer:






