Because of the complex concentration functionality of the (m)-values, VLE calculations in general require iterative procedures suited
Question:
Because of the complex concentration functionality of the \(m\)-values, VLE calculations in general require iterative procedures suited only to computer solutions. However, in the case of mixtures of light hydrocarbons, we may assume as a reasonable approximation that both the liquid and the vapor phases are ideal. This allows \(m\)-values for light hydrocarbons to be calculated and correlated as functions of \(T\) and \(P\). Approximate values can be determined from the monographs prepared by DePriester (1953). The DePriester charts have been fit to the following equation (McWilliams, 1973):
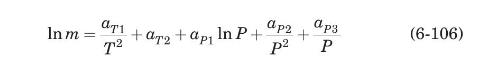
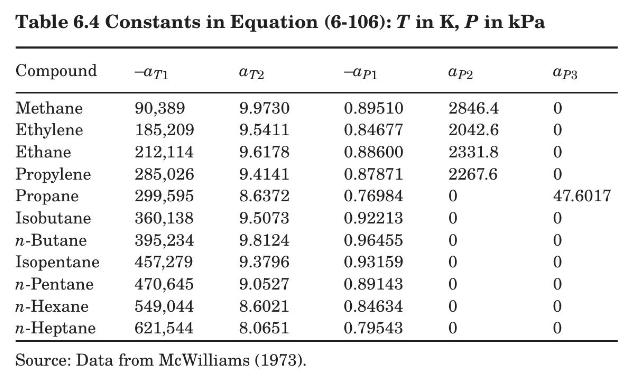
where \(T\) is in \(\mathrm{K}\) and \(P\) is in \(\mathrm{kPa}\). The constants \(a_{T 1}, a_{T 2}, a_{P 1}, a_{P 2}\), and \(a_{P 3}\) are given in Table 6.4. This equation is valid for temperatures from \(200 \mathrm{~K}\) to \(473 \mathrm{~K}\), and pressures from \(101.3 \mathrm{kPa}\) to \(6000 \mathrm{kPa}\).
What is the bubble point of a mixture that is \(15 \mathrm{~mol} \%\) isopentane (A), \(30 \mathrm{~mol} \%\) \(n\)-pentane (B), and \(55 \mathrm{~mol} \% n\)-hexane (C)? Calculate the composition of the first bubble of vapor. The pressure is \(1.0 \mathrm{~atm}\).
Step by Step Answer:






