Calculate the ideal break time for the ion exchanger of Problem 9.11. Data From Problem 9.11:- A
Question:
Calculate the ideal break time for the ion exchanger of Problem 9.11.
Data From Problem 9.11:-
A bed of ion-exchange beads \(1.5 \mathrm{~mm}\) in diameter is used to deionize water at \(293 \mathrm{~K}\) with a superficial velocity of \(0.6 \mathrm{~cm} / \mathrm{s}\). The feed concentration is \(0.02 \mathrm{M} \mathrm{NaCl}\), and the resin has an equilibrium capacity of \(2.4 \mathrm{eq} / \mathrm{L}\) of resin bed. The porosity of the 2 -m-depth bed is 0.35 . For monovalent ions, the effective diffusivity in the pores of the resin is about one-tenth of the normal liquid diffusivity (McCabe et al., 2005). Calculate the volumetric overall mass-transfer coefficient, \(K_{c} a\), and the number of mass-transfer units, \(N\). Use equation (1-141) to calculate the diffusivity of \(\mathrm{NaCl}\) in water.
Equation 1-141:-
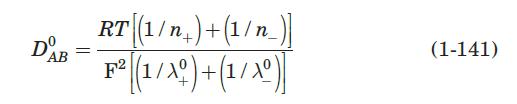
Step by Step Answer:






