Cavatorta et al. (1999) studied the electrochemical reduction of ferricyanide ions, (left{mathrm{Fe}(mathrm{CN})_{6} ight}^{-3}), to ferrocyanide, (left{mathrm{Fe}(mathrm{CN})_{6} ight}^{-4}),
Question:
Cavatorta et al. (1999) studied the electrochemical reduction of ferricyanide ions, \(\left\{\mathrm{Fe}(\mathrm{CN})_{6}\right\}^{-3}\), to ferrocyanide, \(\left\{\mathrm{Fe}(\mathrm{CN})_{6}\right\}^{-4}\), in aqueous alkaline solutions. They studied different arrangements of packed columns, including fluidized beds. The fluidized bed experiments were performed in a 5 -cm-ID circular column, \(75 \mathrm{~cm}\) high. The bed was packed with \(0.534-\mathrm{mm}\) spherical glass beads, with a particle density of \(2.612 \mathrm{~g} / \mathrm{cm}^{3}\). The properties of the aqueous solutions were density \(=1083 \mathrm{~kg} / \mathrm{m}^{3}\), viscosity \(=1.30 \mathrm{cP}\), diffusivity \(=5.90 \times 10^{-10} \mathrm{~m}^{2} / \mathrm{s}\). They found that the porosity of the fluidized bed, \(\varepsilon\), could be correlated with the superficial liquid velocity based on the empty tube, \(v_{s}\), through
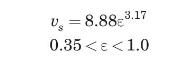
where \(v_{s}\) is in \(\mathrm{cm} / \mathrm{s}\).
(a) Using equation (2-91), estimate the mass-transfer coefficient, \(k_{L}\), if the porosity of the bed is \(60 \%\).
(b) Cavatorta et al. (1999) proposed the following correlation to estimate the mass-transfer coefficient for their fluidized bed experimental runs:
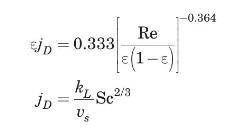
where Re is based on the empty tube velocity. Using this correlation, estimate the mass-transfer coefficient, \(k_{L}\), if the porosity of the bed is \(60 \%\). Compare your result to that of part (a).
Data From Equation 2-91:-
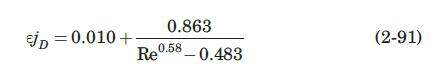
Step by Step Answer:






