Consider the extraction process of Problem 7.3. Calculate the total amount of solvent required if the extraction
Question:
Consider the extraction process of Problem 7.3. Calculate the total amount of solvent required if the extraction is done in a crosscurrent cascade consisting of 5 ideal stages. Use equation (3-89) from Problem 3.22.
Data From Problem 7.3:-
A feed of 13,500 kg/h consists of 8 wt% acetic acid in water. Acetic acid will be removed from the solution by extraction with pure methyl isobutyl ketone at 298 K. If the raffinate is to contain only 1 wt% of acetic acid, estimate the kilograms/hour of solvent required if a single equilibrium stage is used. Assume that water and methyl isobutyl ketone are insoluble. For this system, KD = 0.657 kg water/kg methyl isobutyl ketone (Perry and Chilton, 1973).
Data From Problem 3.22:-
The drying and liquid–liquid extraction operations described in Problems 3.20 and 3.21, respectively, are examples of a flow configuration called a cross-flow cascade. Figure 3.30 is a schematic diagram of a cross-flow cascade of ideal stages. Each stage is represented by a circle, and within each stage mass transfer occurs as if in cocurrent flow. The L phase flows from one stage to the next, being contacted in each stage by a fresh V phase. If the equilibrium-distribution curve of the cross-flow cascade is everywhere straight and of slope m, it can be shown that (Treybal, 1980)
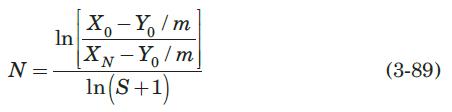
where S is the stripping factor, mVS/LS, constant for all stages, and N is the total number of stages. Solve Problem (3.21) using equation (3-89) and compare the results obtained by both methods.
Data From Problem 3.20:-
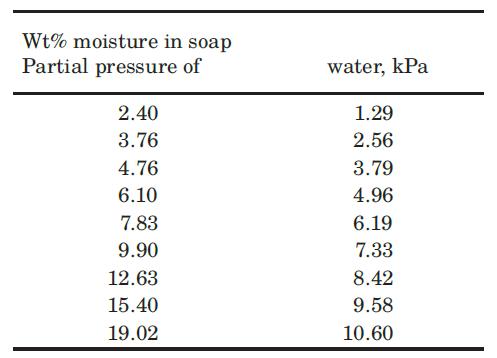
It is desired to dry 10 kg of soap from 20% moisture by weight to no more than 6% moisture by contact with hot air. The wet soap is placed in a vessel containing 8.06 m3 of air at 350 K and 1 atm, and a water-vapor partial pressure of 1.6 kPa. The system is allowed to reach equilibrium, and then the air in the vessel is entirely replaced by fresh air of the original moisture content and temperature. How many times must the process be repeated in order to reach the specified soap moisture content of no more than 6%? When this soap is exposed to air at 350 K and 1 atm, the equilibrium distribution of moisture between the air and the soap is as follows:
Data From Problem 3.21:-
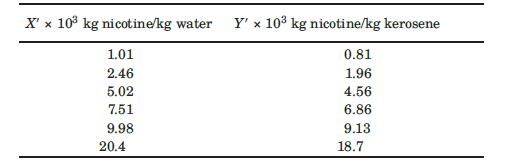
Nicotine in a water solution containing 2% nicotine is to be extracted with kerosene at 293 K. Water and kerosene are essentially insoluble in each other. Determine the percentage extraction of nicotine if 100 kg of the feed solution is extracted in a sequence of four batch ideal extractions using 49.0 kg fresh, pure kerosene each. The equilibrium data are given as follows (Claffey et al., 1950):
Data From Equation 3-89:-
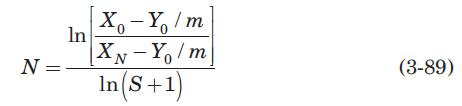
Step by Step Answer:






