To illustrate the slowness of molecular diffusion, an environmental engineering professor claims that one could place a
Question:
To illustrate the slowness of molecular diffusion, an environmental engineering professor claims that one could place a droplet of water containing \(100 \mu \mathrm{g}\) of \(n\)-hexadecane into the middle of a stagnant water-filled pipe ( 4 - \(\mathrm{cm}\) diameter), and that it would take at least a week until the maximum concentration anywhere in the pipe drops to \(1.0 \mathrm{mg} / \mathrm{L}\). How long does it really take? The water temperature is \(298 \mathrm{~K}\). At this temperature, the molecular diffusivity of \(n\)-hexadecane in water is \(4.13 \times 10^{-6} \mathrm{~cm}^{2} / \mathrm{s}\) (Yaws, 2009).
Consider the symmetric propagation by molecular diffusion of a chemical with total mass \(m^{\prime \prime}\) per unit area into an infinite space \((x= \pm \infty)\). At time \(t=0\), this compound is all concentrated at \(x=0\). It can be shown that application of Fick's second law to this case gives the following expression for concentration of the chemical as function of time and position (Scwarzenbach et al., 2017):
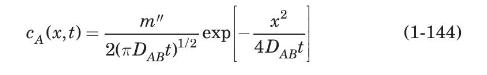
Step by Step Answer:






