Use the Gilliland correlation to estimate the number of ideal stages required for the separation of Example
Question:
Use the Gilliland correlation to estimate the number of ideal stages required for the separation of Example 6.4. Assume that, for the system benzene and toluene at atmospheric pressure, the relative volatility is constant at \(\alpha=2.5\). Use the results of Problems 6.15 and 6.16. Use the Kirkbride equation to estimate the optimum feed-stage location.
Data From Example 6.4:-
A trayed tower operating at 1 atm is to be designed to continuously distill 200 kmol/h (55.6 mol/s) of a binary mixture of 60 mol% benzene, 40 mol% toluene. A liquid distillate and a liquid bottoms product of 95 mol% and 5 mol% benzene, respectively, are to be produced. Before entering the column, the feed—originally at 298 K—is flash-vaporized at 1 atm to produce an equimolal vapor–liquid mixture (VF/F = LF/F = 0.5). A reflux ratio 30% above the minimum is specified. Calculate:
(a) quantity of the products;
(b) minimum number of theoretical stages, Nmin;
(c) minimum reflux ratio;
(d) number of equilibrium stages and the optimal location of the feed stage for the reflux ratio specified; and
(e) thermal load of the condenser, reboiler, and feed preheater.
Data From Problem 6.15:-
For continuous distillation of a binary mixture of constant relative volatility, Fenske equation (6-58) can be used to estimate the minimum number of equilibrium stages required for the given separation, \(N_{\min }\).
Data From Problem 6.16:-
For continuous distillation of a binary mixture of constant relative volatility, the minimum reflux ratio can be determined analytically from the following equation (Treybal, 1980):
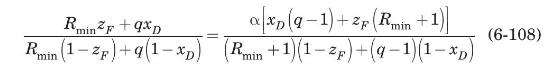
Use (6-108) to estimate \(R_{\min }\) for distillation of the benzene-toluene mixture of Example 6.4. Assume that, for this system at \(1 \mathrm{~atm}\), the relative volatility is constant at \(\alpha=2.5\).
Step by Step Answer:






