We wish to remove acetic acid from water using pure isopropyl ether as solvent. The operation is
Question:
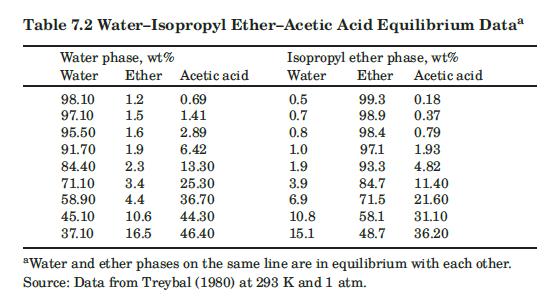
We wish to remove acetic acid from water using pure isopropyl ether as solvent. The operation is at \(293 \mathrm{~K}\) and \(1 \mathrm{~atm}\) (see Table 7.2). The feed is \(45 \mathrm{wt} \%\) acetic acid and \(55 \mathrm{wt} \%\) water. The feed flow rate is \(2000 \mathrm{~kg} / \mathrm{h}\). A multistage countercurrent extraction cascade is used to produce a final extract that is \(20 \mathrm{wt} \%\) acetic acid and a final raffinate that is also \(20 \mathrm{wt} \%\) acetic acid. Calculate how much solvent and how many equilibrium stages are required.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





