(a) Confirm that H 2 Os 3 (CO) 11 has sufficient valence electrons to adopt a triangular...
Question:
(a) Confirm that H2Os3(CO)11 has sufficient valence electrons to adopt a triangular metal framework. Do the modes of bonding of the CO and H ligands affect the total valence electron count? Comment on the fact that H2Os3(CO)10 also has a triangular Os3-core.
(b) The 1H NMR spectrum of H2Os3(CO)11 in deuterated toluene at 183K shows two major signals (relative integrals 1 : 1) at δ–10.46 and –20.25 ppm; both are doublets with J = 2:3 Hz. The signals are assigned to the terminal and bridging H atoms, respectively, in the structure shown below:
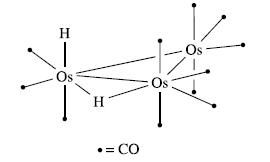
The 1H NMR spectrum also exhibits two pairs of low-intensity signals: δ–12.53 and –18.40 ppm (both doublets, J = 17:1 Hz) and δ –8.64 and –19.42 ppm (no coupling resolved). These signals are assigned to two other isomers of H2Os3(CO)11. From other NMR spectroscopic experiments, it is possible to show that the two H atoms in each isomer are attached to the same Os centre. Suggest structures for the minor isomers that are consistent with the NMR spectroscopic data.
Step by Step Answer:






