(a) Estimate the value of f H(WCl 2 ) assuming it to be an ionic compound....
Question:
(a) Estimate the value of ΔfH°(WCl2) assuming it to be an ionic compound. Comment on any assumptions made. [Data needed in addition to those in Tables 21.1, 22.1 and the Appendices: ΔfH°(CrCl2) = −397 kJ mol−1].
(b) What does your answer to (a) tell you about the likelihood of WCl2 being ionic?
Table 21.1.
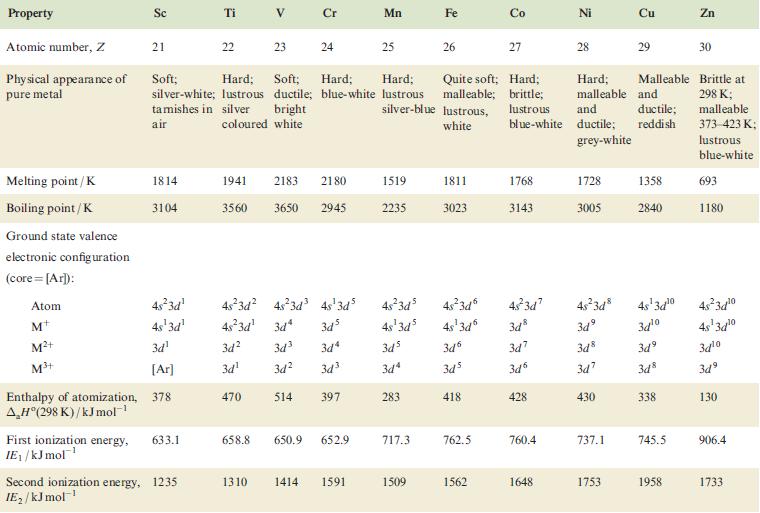
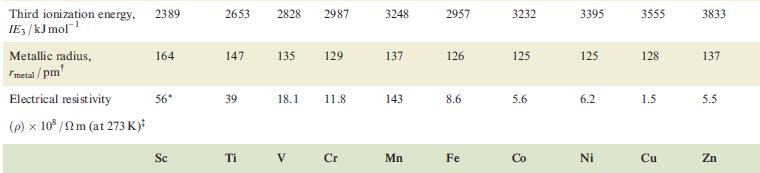
Table 22.1
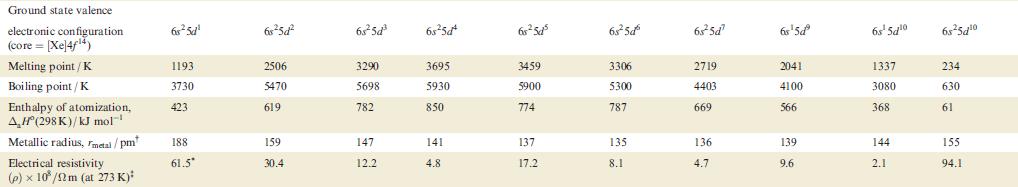
Transcribed Image Text:
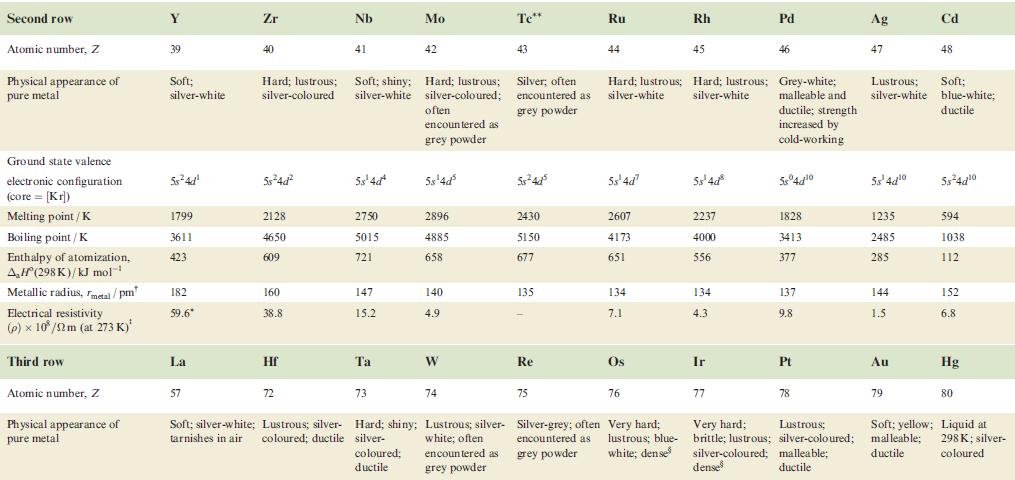
Property Atomic number, Z Physical appearance of pure metal Melting point / K Boiling point/K Ground state valence electronic configuration (core=[Ar]): Atom M+ M²+ M³+ Enthalpy of atomization, AH (298 K)/kJ mol-¹ Sc 21 1814 3104 4s²3d¹ 4s¹3d¹ 3d¹ [Ar] 378 First ionization energy, 633.1 IE₁/kJ mol™¹ Ti Second ionization energy, 1235 IE₂/kJ mol-¹ 22 V 23 Cr 24 1941 2183 2180 3560 3650 2945 3d² 3d³ 3d¹ 470 Soft; Hard; Soft; Hard; Hard; Quite soft; Hard; silver-white; lustrous ductile; blue-white lustrous malleable; brittle; tamishes in silver bright silver-blue lustrous, lustrous air coloured white white blue-white 48²3d² 4s²3d³ 4s¹3d5 4s²3d¹3d4 345 3d4 3d² 3d³ 514 397 658.8 650.9 652.9 Mn 1310 1414 1591 25 1519 2235 Fe 717.3 26 1509 1811 3023 3d6 3d5 418 Co 762.5 27 1562 4s²3d³ 48²3d 45-3d7 4s²3d8 4s¹3d³ 4s¹3d6 3d8 зая 3d³ 3d7 348 3d4 3d6 3d7 283 1768 3143 428 760.4 Ni 1648 28 1728 3005 Hard; Malleable and malleable and ductile; ductile; reddish grey-white 430 737.1 Cu 1753 29 1358 2840 4s¹3d¹⁰ 34¹⁰ 3d9 3d8 338 745.5 1958 Zn 30 Brittle at 298 K; malleable 373-423 K; lustrous blue-white 693 1180 4s²3d¹0 4s¹3d¹0 3d¹0 3d⁹ 130 906.4 1733
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 80% (5 reviews)
To estimate the value of fHWCl2 as if it were an ionic compound ...View the full answer

Answered By

Rehab Rahim
I am well versed in communicating and teaching in areas of all business subjects. I have helped many students in different ways from answering answers to writing their academic papers.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
The first quarter of 2008 had not yet ended and Steve Savage already knew the company would surpass the projected $22 million in revenues for the year. He and the management team had doubled sales...
-
Read the comment letter below written by Micron's CFO to the FASB dated June 30, 2004 and answer the following question. Director of Major Projects Financial Accounting Standards Board 401...
-
In our development of consumer theory, we made a big point about the fact that neoclassical economics does not put much stock in the idea of cardinally measuring utility (in terms of units of...
-
Night By Elie Wiesel The Holocaust - Why did the members of Sighets Jewish community refuse to believe their horrible situation? (Moshe the Beadle and Madame Schachter portending the horrors that...
-
Expando, Inc., is considering the possibility of building an additional factory that would produce a new addition to its product line. The company is currently considering two options. The first is a...
-
What is an ADO DataSet?
-
Write a Monte Carlo code for a system of \(N\) hard spheres of diameter \(D\) on a one-dimensional ring of length \(L\) with periodic boundary conditions. Calculate the pair correlation function and...
-
On July 31, 2012, Mexico Company paid $3,000,000 to acquire all of the common stock of Conchita Incorporated, which became a division of Mexico. Conchita reported the following balance sheet at the...
-
Jobs R Us, Inc. Is a recruiting firm that specializes in post- college placement in the finance industry. Its clients are currently concentrated in the North-Eastern United States lt is contemplating...
-
Apply the translation theorem to find the Laplace transforms of the functions in Problems 1 through 4. f(t) = e-1/2 cos 2 (1 ) gr
-
Briefly discuss trends in (a) Metallic radii and (b) Values of a H(298 K) for the d-block metals.
-
(a) Suppose you are given three springs with respective stiffnesses c = 1. c = 2, c = 3. In what order should you connect them to three masses and a top support so that the bottom mass goes down the...
-
Write an interactive Java program that prompts for and reads for a person: the year of birth, the last name, and his first and middle initials. It then computes and displays the person's age in the...
-
f'(x) = f'(1) = Let f(x) = (x + 5x +4) 0
-
A small village on the Bintangor river, a tributary of the Sarawak in Malaysia, is considering installing a 3 6 0 kW hydroelectric plant, for the benefit of its 9 9 residents. The capital cost will...
-
5. Shown below is a WHILE-DO loop statement which replaces the value stored in x with the absolute value of x. Using r0 to represent x, implement the IF-THEN statement in ARM assembly language. while...
-
Discuss the backoff policies used in our implementation. Does it make sense to use the same shared Backoff object for both pushes and pops in our LockFreeStack object? How else could we structure the...
-
Finch Construction Company provides its employees who are carpenters with all of the required tools. However, the company believes that this has led to some employees not taking care of the tools and...
-
CdF2 (s) Cd+ (aq) + 2 F- (aq) 1. A saturated solution of CdF2 is prepared. The equilibrium in the solution is represented above. In the solution [Cd+] eq = 0.0585 M and [F-] eq = 0.117 M. a....
-
State the effect on the rate of dissociatively activated reactions of Rh(III) complexes of (a) An increase in the overall charge on the complex, (b) Changing the leaving group from NO 3 to Cl , (c)...
-
Put in order of increasing rate of substitution by H 2 O the complexes (a) [Co(NH 3 ) 6 ] 3+ , (b) [Rh(NH 3 ) 6 ] 3+ , (c) [Ir(NH 3 ) 6 ] 3+ , (d) [Mn(OH 2 ) 6 ] 2+ , (e) [Ni(OH 2 ) 6 ] 2+ .
-
Predict the products of the following reactions: (a) [Pt(PR 3 ) 4 ] 2+ + 2Cl (b) [PtCl 4 ] 2 + 2PR 3 (c) cis-[Pt(NH 3 ) 2 (py) 2 ] 2+ + 2Cl
-
1. Convert the following C program to MIPS program. Assuming that i, j, k, f, are stored in registers $50, $s1, $s2, $s3 already. The base address of arrays A and B are in registers $54 and $s5,...
-
Demonstrate the utility of database automation. Describe commonly used database automation tools. Describe SQL server integration services (SSIS) data migration software, SQL server analysis services...
-
2. Consider the following C program: i=0; do { c[i]=a[i]-b[i]; i++; } while (i

Study smarter with the SolutionInn App


