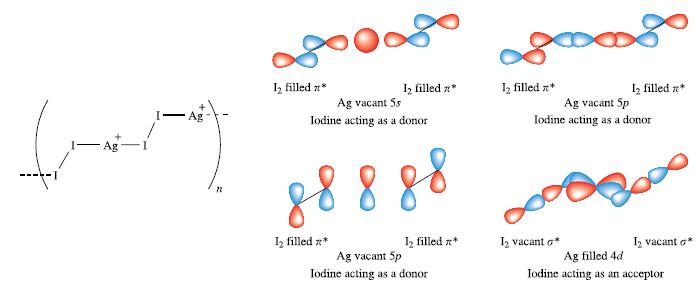
(a) Figure 17.7 showed the structure of [(AgI 2 )n] n+ and an MO scheme for the...
Question:
(a) Figure 17.7 showed the structure of [(AgI2)n]n+ and an MO scheme for the bonding. The bonding may also be represented using the valence bond approach. The diagram below illustrates the positive charge localized on Ag+ centres. Use this as a starting point to draw a set of resonance structures which illustrate I2 acting as a charge donor. How does this compare with a VB scheme for the bonding in [I5]+?
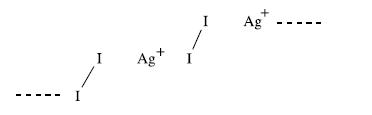
(b) When I2 reacts with SbF5 in liquid SO2, the compound [I4][Sb3F14][SbF6] is formed. Explain what happens in this reaction, and draw the structures of the ions present in the product. Assign oxidation states to each atom in the product.
Figure 17.7.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





