(a) The complex shown below is the first example of a Pd(IV) complex containing a nitrosyl ligand...
Question:
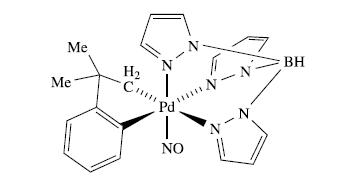
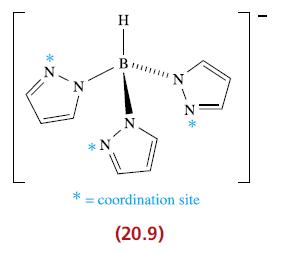
(a) The complex shown below is the first example of a Pd(IV) complex containing a nitrosyl ligand (see also structure 20.9 for another view of the tridentate ligand). On the basis of the assignment of an oxidation state of +4 for Pd, what formal charge does the nitrosyl ligand carry? In view of your answer, comment on the fact that structural and spectroscopic data for the complex include the following parameters: —Pd—N—O = 118°, N—O = 115 pm, ν̅ (NO) = 1650 cm−1 (a strong absorption).
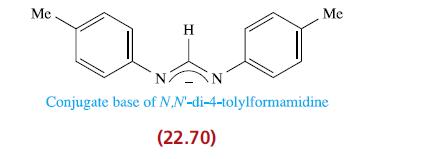
(b) The reaction of equimolar equivalents of [Bu4N]2[C2O4] with [cis-Mo2(m-L)2(MeCN)4][BF4]2 where L− is a formamidine ligand closely related to 22.70 leads to a neutral compound A which is a so-called ‘molecular square’. Bearing in mind the structure of [C2O4]2−, suggest a structure for A. This compound might also be considered as a [4 + 4] assembly. What experimental techniques would be useful in distinguishing compound A from a possible [3 + 3] product?
Step by Step Answer:






