(a) The reaction: occurs by a dissociative mechanism and the first order rate constants, k 1 ,...
Question:
(a) The reaction:
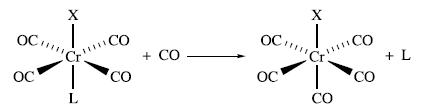
occurs by a dissociative mechanism and the first order rate constants, k1, vary with the nature of substituent X as follows: CO 3 ≈ P(OPh)3nBu3: Comment on these data.
(b) The ligand, L, shown below forms the complex [PtLCl]+ which reacts with pyridine to give [PtL(py)]2+.
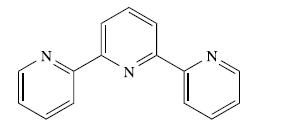
The observed rate constant, kobs, can be written as:
![]()
What conformational change must ligand L make before complex formation? Explain the origins of the two terms in the expression for kobs.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





