(a) The value of eff for [CoF 6 ] 3 is 5.63 B . Explain...
Question:
(a) The value of μeff for [CoF6]3− is 5.63 μB. Explain why this value does not agree with the value for μ calculated from the spin-only formula.
(b) By using a simple MO approach, rationalize why one-electron oxidation of the bridging ligand in [(CN)5CoOOCo(CN)5]6− leads to a shortening of the O—O bond.
(c) Salts of which of the following complex ions might be expected to be formed as racemates:
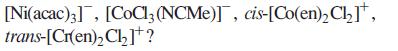
Transcribed Image Text:
[Ni(acac)3], [CoCl3 (NCMe)], cis-[Co(en)₂Cl₂], trans-[Cr(en)₂Cl₂]¹?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (6 reviews)
a The value of eff effective magnetic moment for CoF63 is given as 563 B Bohr magnetons However this value does not agree with the value for magnetic moment calculated from the spinonly formula The sp...View the full answer

Answered By

Aketch Cindy Sunday
I am a certified tutor with over two years of experience tutoring . I have a passion for helping students learn and grow, and I firmly believe that every student has the potential to be successful. I have a wide range of experience working with students of all ages and abilities, and I am confident that I can help students succeed in school.
I have experience working with students who have a wide range of abilities. I have also worked with gifted and talented students, and I am familiar with a variety of enrichment and acceleration strategies.
I am a patient and supportive tutor who is dedicated to helping my students reach their full potential. Thank you for your time and consideration.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
In the Rutherford scattering experiment, the minimum distance of approach for the alpha particle is given by Eq. 29.1. Explain why this distance does not necessarily represent the nuclear radius. Is...
-
Explain why this fact does not contradict the main premise of this section with regard to the uniqueness of the stable distribution? Show that And
-
As shown in Example 2, is not a regular stochastic matrix. Show that acts like a stable distribution for this matrix, and explain why this fact does not contradict the main premise of this section?...
-
Companies use off-balance sheet accounting so that they do not have to include certain assets and liabilities in their financial statements. Off-balance sheet accounting is often used to make the...
-
You work for Nokia in its global cell phone group. You have been made project manager for the design of a new cell phone. Your supervisors have already scoped the project so you have a list showing...
-
Figure P.6.34 shows the distribution of light corresponding to the image arising when a monochromatic point source illuminates two different optical systems each having only one type of aberration....
-
Can you create a graphic of the Fairmont payroll systems company expenses that highlight general accounting clerk, Mary Perez, has company expenses for FICA (social security), Medicare and 401K,...
-
Error Corrections and Accounting Changes Penn Company is in the process of adjusting and correcting its books at the end of 2010. In reviewing its records, the following information is compiled. 1....
-
Two years after the first round XMP GmbH receives a second offer form B-Capital. B-Capital offers to invest 12,500,000.00 at a 60,000,000.00 pre-money valuation. What is the founders' post-round...
-
(a) When [CN] is added to aqueous Ni 2+ ions, a green precipitate forms; if excess KCN is added, the precipitate dissolves to give a yellow solution and at high concentrations of [CN] , the...
-
Give explanations for the following observations. (a) The complex [Co(en) 2 Cl 2 ] 2 [CoCl 4 ] has a room temperature magnetic moment of 3.71 eff . (b) The room temperature magnetic moment of [CoI 4...
-
Is technological change a good thing? List some major benefits and major problems of technology if you could stop technological change, would you want to do so, and why?
-
Consider the Sequential Intercept Model and your area of interest in criminal justice, whether it is in the courts, police department, probation, parole, or detention. What does "intake" look like?...
-
(b) Consider the following function: F(x,y,z)= x'yz + xy'z' + xy' Now draw the circuit diagram of the above function using only NAND-gate.
-
Explain the behavior of the Java code for the bounded buffer problem if the keyword synchronized is removed from the put operation (line 28 of Figure 13.6). (1) import java.io.*; (2) class...
-
An organization is considering a capital investment in the new equipment. The estimated cash flows are as follows. Year Cash flow 0 (240,000) 1 80,000 2 120,000 3 70,000 4 40,000 5 20,000 The...
-
Based on the following information, what is the amount of Beth's self-employment income? Salary from her S corp $15000 Partnership income (general partner) $8000 Corporate director's fees $1500...
-
Veronica is a key employee of Perdiz Corporation, an aerospace engineering concern located in Seattle. Perdiz would like to establish an office on the east coast of Florida and wants Veronica to be...
-
1-Stern observed all of the following results EXCEPT _______ in his experiment. A-one of the recombinant phenotypes was associated with an X chromosome of normal length B-the number of car, B+ male...
-
For each of the following cases use the information given to determine whether or not the equilibrium will favor products over reactants: (a) A reaction with K eq = 1.2 (b) A reaction with K eq = 0.2...
-
Propose a mechanism for the following transformation: CI HCI
-
Identify the relationship between the following two compounds.
-
Demonstrate the utility of database automation. Describe commonly used database automation tools. Describe SQL server integration services (SSIS) data migration software, SQL server analysis services...
-
Step #1: Brainstorm your purpose Purpose = talents + passions + skills/expertise + values Identify at least 4 attributes for each area of your personal and professional talents, passions,...
-
How do chromosomal abnormalities, such as aneuploidy, translocations, inversions, and deletions, arise during meiosis and mitosis, and what are the clinical manifestations and diagnostic implications...

Study smarter with the SolutionInn App


