(a) Use Wades rules to rationalize the fact that in [Pd@Bi 10 ] 4+ , the Bi...
Question:
(a) Use Wade’s rules to rationalize the fact that in [Pd@Bi10]4+, the Bi atoms are arranged in a pentagonal antiprism. How is this structure related to that of [Pd@Pb12]2−?
(b) At 298 K, ammonium perchlorate decomposes according to the equation:
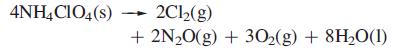
Determine ΔrG°(298 K) for this decomposition if ΔfG°(298 K) of N2O(g), H2O(l) and NH4ClO4(s) are +104, –237 and –89 kJ mol–1. What part does entropy play in the reaction?
Transcribed Image Text:
4NH4CIO4(s) 2Cl₂(g) + 2N₂O(g) + 30₂(g) + 8H₂O(1)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (4 reviews)
ANSWER a Wades rules are a set of guidelines used to predict the structure of polyhedral boranes and metal cluster compounds based on the number of sk...View the full answer

Answered By

Collins Njuguna
I graduated from Maseno University with a Bachelor of Science in Applied Statistics. After graduation, I started tutoring students in mathematics. My experience in mathematics education is extensive and varied. I have taught a wide range of topics, including algebra, geometry, trigonometry, calculus, statistics, probability, and computer science. I have also worked with students of all ages and backgrounds, from elementary school to college.
My teaching method is based on the idea of hands-on learning. I believe that students learn best when they are actively engaged in the learning process, so I focus on giving students the tools they need to explore the material on their own. I also emphasize the importance of practice and review, as these are essential for mastering math concepts.
I have also developed several online and in-person courses on mathematics. My courses are designed to help students learn mathematics in an efficient and comprehensive way, and I use a variety of activities and exercises to ensure that my students are engaged and motivated.
Overall, my passion for mathematics and teaching has allowed me to be a successful tutor and educator in the field. I am confident that my experience will help your students master the mathematics they need to succeed.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
A simplified version of a commercial nuclear reactor involves fissile material such as enriched uranium 12 and a moderator such as graphite, both of which will be assumed in this exercise. Slow...
-
According to Van Luling what are some examples of how Trump's own rhetoric invokes the image and iconography of the Kong myth? In October of 2016, Daily Show host Trevor Noah compared Trump to Kong....
-
Please write an summary according to the article. Please don't copy from others and write full view of article in the summary. Value creation. Wealth creation. These are really powerful words. Maybe...
-
1. Read the following article about the new circle with disney product and answer the following questions. in doing so, take on the role as the marketing manager responsible for the product and...
-
John Marshall is employed as a bank loan officer for First State Bank. He is comparing two companies that have applied for loans, and he wants your help in evaluating those companies. The two...
-
In ADSL service, there is a single UTP pair running from the end office switch to the individual household. In cable in the neighborhood is shared by many subscribers. Yet, typically, cable modem...
-
Some of the prior interviews suggest that the company is so cheap that they wont pay people what they are worth. Have you had similar experiences?
-
Accounting for bonds using amortized cost measurement based on the historical market interest rate. Several years ago, Huergo Dooley Corporation (HDC) issued $2,000,000 face value, 8% semiannual...
-
The following data is provided for Garcon Company and Pepper Company for the year ended December 31. Finished goods inventory, beginning Work in process inventory, beginning Raw materials inventory,...
-
Predict the structures of (a) [NF 4 ] + ; (b) [N 2 F 3 ] + ; (c) NH 2 OH; (d) SPCl 3 ; (e) PCl 3 F 2 .
-
Deduce what you can about the nature of the following reactions. (a) One mole of NH 2 OH reacts with two moles of Ti(III) in the presence of excess alkali, and the Ti(III) is converted to Ti(IV). (b)...
-
Name the four principal ways a structural geologist can learn about structural geology and rock deformation. How would you rank them?
-
What is HTML and what is its role in web development? Question 1 2 1 2 options: A high - level programming language interpreted by a browser. The standard markup language used for creating web pages...
-
The size of any arrays is determined using The member variable called size O The method length() O The member variable called length The method size() returns the number of cells
-
Positional parameters are a . . special variables inside the script b . . special variables for setting arguments in the command line c . . special patterns and symbols from command line d . . group...
-
21) Dijkstra's Algorithm is used to solve ..... problems. a) Shortest path from any node to any other node on the graph. (All pair shortest path) b) Shortest path from a given sounrce node to any...
-
How have recent developments in information technology made the operations of businesses more effective? By reducing the need for business processes By automating tasks that were previously manual By...
-
Find the range, the variance, and the standard deviation of the data set. 0.7, 0.8, 0.1, -0.7, -0.7, 1.6, 0.2, -0.5, -0.4, -1.3
-
Suppose Green Network Energy needs to raise money to finance its new manufacturing facility, but their CFO does not think the company is financially capable of making the periodic interest payments...
-
In November 2006 the former KGB agent Alexander Litvinenko was found to have been poisoned by radioactive polonium-210. Write a review of the chemical and radiological properties of Po and discuss...
-
In their paper Formation of tellurium nanotubes through concentration depletion at the surfaces of seeds (Adv. Mater., 2002, 14, 279), B. Mayers and Y. Xia describe the synthesis of tellurium...
-
Correct any inaccuracies in the following statements and, after correction: (a) Elements in the middle of Group 16 are easier to oxidize to the group oxidation number than are the lightest and...
-
The US government is aware of the economy's sensitivity to supply chain issues and has initiated a response. Using your own independent research, explain the actions the US government has taken to...
-
Suppose that between 1995 and 2013, US external that rose from a percent of GDP to over 29% of GDP this increase in external debt owed to foreign entities ?Explain
-
Suppose that consumers become pessimistic about the overall economy because of partisan politics. What would you expect to happen to the aggregate demand curve?

Study smarter with the SolutionInn App


